Properties & Tempertature Effects
Polymer Properties
When considering a polymer for use in any application it is essential to understand the behaviour of the polymer with respect to variations in temperature.
Unlike metals or ceramics that have high melting points, polymers can undergo a substantial number of changes in state as they pass through a temperature range of between -20°C and 200°C.
Dependent on its temperature, a polymer can be plastic, viscoelastic, leathery, rubbery, or viscous. Each change of state can affect the polymer's strength and stiffness by a factor of up to 103.
Temperature Effects
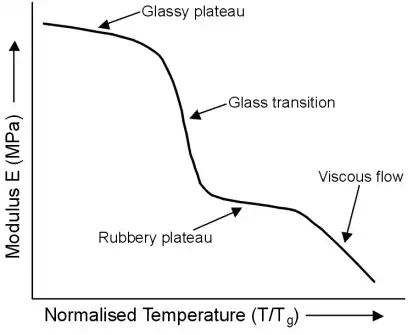
The following graph is a representation of changes in a polymer's stiffness, or modulus, with respect to temperature.
Temperature is normalised against the glass transition temperature, Tg, as the behaviour of polymers depends on how close the temperature is to their Tg.
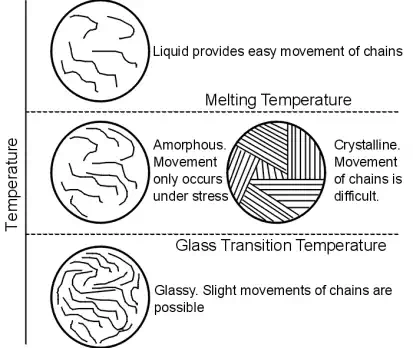
Temperature is an extremely important factor in the design of polymers. This is principally due to the fact that the secondary Van Der Waals forces, which bind the chains together, can be overcome with slight increases in temperature.
An increase in temperature to above its Tg can convert a polymer from a rigid brittle material to a viscoelastic material, that exhibits both elastic and viscous qualities.