COMPARISON BETWEEN POLYMER TYPE AND KINETICS OF POLYMERIZATION
There is a large but not total overlap between the terms condensation polymers and stepwise kinetics and the terms addition (or sometimes the term vinyl) polymers and chain kinetics. In this section we will describe briefly each of these four terms and illustrate their similarities and differences. The terms addition and condensation polymers were first proposed by Carothers and are based on whether the repeat unit of the polymer contains the same atoms as the monomer. An addition polymer has the same atoms as the monomer in its repeat unit
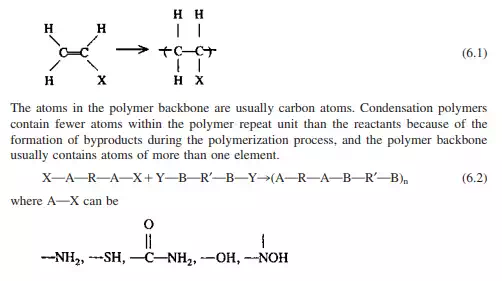
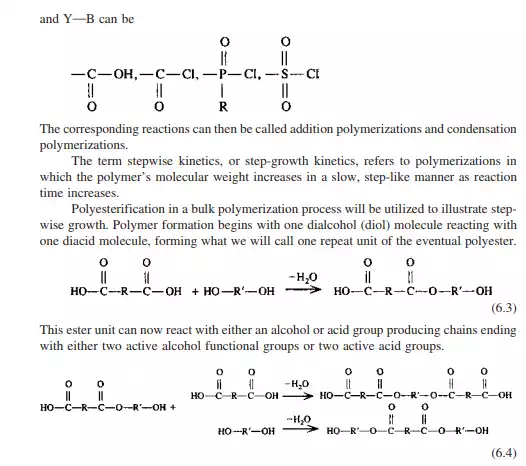
The chain with the two alcohol end groups can now condense with a molecule containing an acid end group, while the molecule with two acid end groups can react with a molecule containing an alcohol functional group. This reaction continues through the monomer matrix wherever molecules of the correct functionality having the necessary energy of activation and correct geometry collide. The net effect is that dimer, trimer, tetramer, etc., molecules are formed as the reaction progresses.
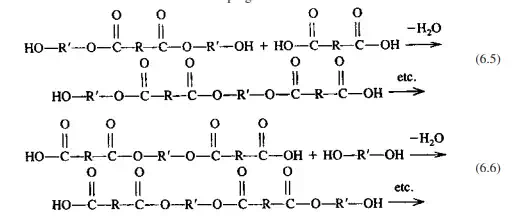
Thus the reactants are consumed with very few long chains being formed throughout the system until the reaction progresses toward total reaction of the chains with themselves. The molecular weight of the total system increases slowly in a stepwise manner. Considering A molecules to be diacid molecules and B to represent dialcohol molecules (diols), we can construct a system containing 10 each of A and B, and assuming a random number of condensations of unlike functional groups, we can calculate changes in degree of polymerization (DP), maximum DP, and the percentage of unreacted monomer (Fig. 6.1). The maximum DP for this system is 10 AB units. For this system, while the percentage of reacted monomer increases rapidly, both system DP and highest DP increase slowly. Figures 6.1 and 6.3 graphically show this stepwise growth as a function of both reaction time and reaction temperature T. It should be noted that the actual plot of molecular