Crystalline or Amorphous
A polymer therefore is a series of long chain molecules composed into a complex arrangement to make a solid.
There are two possible arrangements of the molecular chains. They are either crystalline (a), in the sense that they arrange themselves neatly to have long-range order, or they are amorphous (b) and randomly packed with no discernible order.
While a polymer can be completely amorphous, it will never be completely crystalline but instead will also have regions that are partially amorphous. These structures are referred to as semicrystalline.
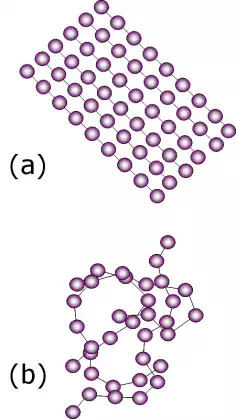
The degree of crystallinity may range from completely amorphous to up to 95% crystalline.
The presence of both amorphous and crystalline regions in a polymer is analogous to a two-phase metal alloy.
Polymers with molecular chains that have large irregularity with respect to attached functional groups find it difficult to arrange into an ordered structure and therefore favour the formation of an amorphous structure.
Viscosity in Crystalline Polymers
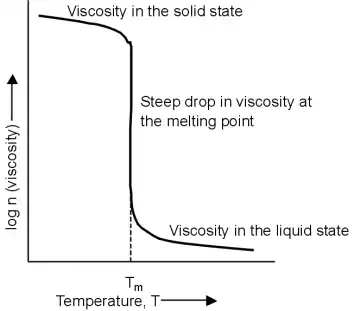
The behaviour of amorphous and crystalline polymers under applied heat is quite different.
In the solid state, a crystalline polymer is rigid with high viscosity. As the polymer is heated to past its melting point the viscosity drops abruptly to that of a flowing liquid. This is similar to ice when it melts into water
Specific Volume in Crystalline Polymers
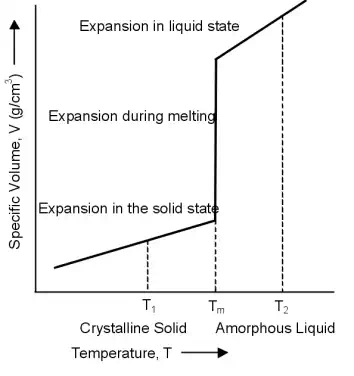
When a crystalline polymer is heated from the solid state it will expand at a constant rate dependent on its coefficient of thermal expansion. At the melting point the polymer undergoes a large increase in volume before once again expanding at a constant rate in the liquid state.
The large expansion, or increase in free volume, occurs as the chains in the crystalline state breakdown into an amorphous structure.