CRYSTALLINE AND AMORPHOUS COMBINATIONS
Most polymers consist of a combination of crystalline and amorphous regions. Even within polymer crystals such as spherulites (Figs. 2.21 and 2.22), the regions between the ordered folded crystalline lamellae are less ordered, approximating amorphous regions. This combination of crystalline and amorphous regions is important for the formation of materials that have both good strength (contributed to largely by the crystalline portions) and some flexibility or “softness” (derived from the amorphous portions). Figure 2.25 contains a space-filled model for polyethylene chains (a total of about 400 units with 5 branches, one longer and four shorter).
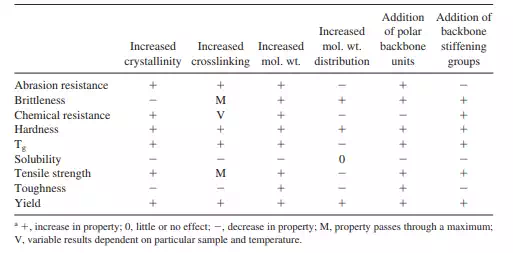
This model of polyethylene (Fig. 2.25) contains a mixture of amorphous and crystalline regions. Note the cavities within the amorphous regions with materials containing a majority of amorphous regions having a greater porosity and consequently a greater diffusion and greater susceptibility to chemical and natural attack. As noted before, materials that contain high amounts of crystalline regions are referred to as being crystalline and are less flexible and stronger—and offer better stability to natural attack by acids and bases, oils, etc. Also as noted before, the amorphous regions give the material flexibility, while the crystalline regions give the material strength. Thus, many materials contain both crystalline and amorphous regions giving the material a balance between strength and flexibility.
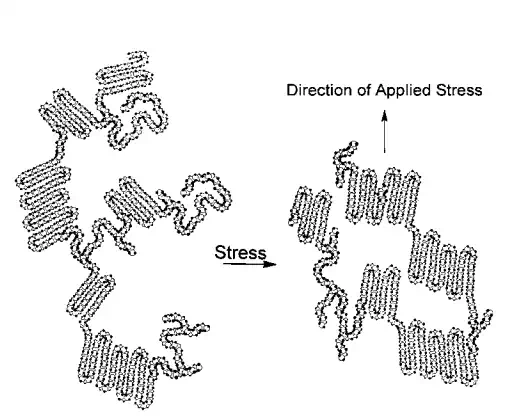
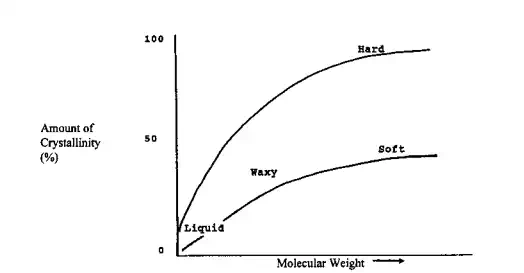
The final properties of a material are then dependent on the molecular structure of that material. Through the use of specific treatment(s) the crystalline/amorphous regions can vary from being largely random to being preferentially oriented in one direction with a greater degree of “crystalline-type” structure when unidirectional stress is applied (Fig. 2.25). Here the amount of free space or volume is less, the overall order is greater and properties associated with these changes are changed. The material will be stronger, have a greater ability to resist attack by acids, bases, oils, and other external agents, and the diffusion of gases and other agents through it is less. Figure 2.26 shows the general relationship between material “hardness/softness” and the proportion that is crystalline for largely linear polymers.
Through the use of specific treatment(s) the crystalline/amorphous regions can vary from being largely random to being preferentially oriented in one direction (Fig. 2.25) and in the proportion of crystalline/amorphous regions. Thus, polymers can be oriented through the unidirectional “pulling” of the bulk material either during the initial synthesis (such as the pulling of fibers as they exit a spinneret) or during the processing phase where preferential application of stress (pulling) in one direction results in the preferential orientation of the chains, including both crystalline and amorphous regions. This preferential orientation results in fibers or bulk material with anisotropic properties, with the
material generally showing greater strength along the axis of applied stress (in the direction of the pull). These crystalline sites may be on a somewhat molecular level involving only a few chains (Fig. 2.25) or they may exist as larger units such as spherulites (Figs. 2.17 and 2.21). The amount of orientation is dependent on a number of factors. Increased mobility of the crystalline and amorphous regions typically results in greater reorientation for a specified applied stress.
Thus, materials with little or no crosslinking, materials with lowered inter- and intramolecular attraction, and materials that are near (or above) their glass transition temperature will respond with greater reorientation (per unit of stress) in comparison to materials where mobility is more limited. Application of increased stress will eventually lead to distortion of both the crystalline (including spherulites) and amorphous regions and finally breakage of primary chains.