POLYMER STRUCTURE–PROPERTY RELATIONSHIPS
Throughout the text we will relate polymer structure to the properties of the polymer. Polymer properties are related not only to the chemical nature of the polymer but also to such factors as extent and distribution of crystallinity, distribution of polymer chain lengths, and nature and amount of additives, such as fillers, reinforcing agents, and plasticizers, to mention only a few. These factors influence essentially all the polymeric properties to some extent, such as hardness, flammability, weatherability, chemical resistance, biological responses, comfort, appearance, dyeability, softening point, electrical properties, stiffness, flex life, moisture retention, etc. Chapters 1 and 12 concentrate on the chemical nature of the polymer itself, whereas Chapters 13 and 14 deal with the nature and effect on polymer properties by addition of plasticizers, fillers, stabilizers, etc. Chapter 17 deals with the application of both the polymers themselves and suitable additives aimed at producing polymers exhibiting desired properties.
Materials must be varied to perform the many tasks required of them in today’s society. Often they must perform them repeatedly and in a “special” manner. We get an idea of what materials can do by looking at some of the behavior of the giant molecules that compose the human body. While a plastic hinge must be able to work thousands of times, the human heart, a complex muscle largely composed of protein polymers (Sec. 10.7), provides about 2.5 billion beats within a lifetime moving oxygen (Sec. 15.10) throughout the approximately 144,000 km of the circulatory system with (some) blood vessels the thickness of hair and delivering about 8000 L of blood every day with little deterioration of the cell walls.
The master design allows nerve impulses to travel within the body at a rate of about 300 m/min; again polymers are the “enabling” material that allows this rapid and precise transfer of nerve impulses. Human bones, again largely composed of polymers, have a strength about five times that of steel. Genes, again polymers, appear to be about 99.9% the same, with the 0.1% functioning to give individuals the variety of size, abilities, etc., that confer uniqueness.
In the synthetic realm, we are beginning to understand and mimic the complexities, strength, and flexibility that are already present in nature (Chapter 10). Here we will deal briefly with the chemical and physical nature of polymeric materials that permits their division into three broad divisions—elastomers or rubbers, fibers, and plastics. Elastomers are high polymers possessing chemical and/or physical crosslinks. For industrial application the “use” temperature must be above Tg (to allow for “chain” mobility), and its normal state (unextended) must be amorphous. The restoring force, after elongation, is largely due to entropy.
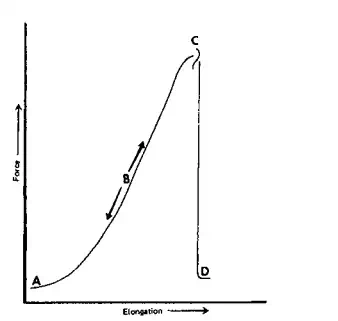
As the material is elongated, the random chains are forced to occupy more ordered positions. On release of the applied force the chains tend to return to a more random state. Gross, actual mobility of chains must be low. The cohesive energy forces between chains should be low to permit rapid, easy expansion. In its extended state a chain should exhibit a high tensile strength, whereas at low extensions it should have a low tensile strength. Crosslinked vinyl polymers often meet the desired property requirements. The material, after deformation, should return to its original shape because of the crosslinking. This property is often referred to as an elastic “memory.” Figure 2.24 illustrates force vs. elongation for a typical elastomer.
As the elastomer is pulled, the largely random chain segments become “stretched out” forming microcrystalline domains. Eventually, most of the chains are part of these microcrystalline domains resulting in further elongation requiring much increased force (stress). This microcrystallization also confers to the elastomer a greater brittleness, eventually resulting in the rubber breaking as additional stress is applied. Fiber properties include high tensile strength and high modulus (high stress for small strains). These can be obtained from high molecular symmetry and high cohesive energies between chains, both requiring a fairly high degree of polymer crystallinity.
Fibers
are normally linear and drawn (oriented) in one direction, producing high
mechanical properties in that direction. Typical condensation polymers, such as
polyester and nylon, often exhibit these properties. If the fiber is to be
ironed, its Tg should be above 200
C, and if it is to be drawn from the melt, its Tg should be below 300
C. Branching and crosslinking are undesirable since they disrupt crystalline
formation, even though a small amount of crosslinking may increase some
physical properties, if effected after the material is drawn and processed.
Products with properties intermediate between elastomers and fibers are grouped together under the heading “plastics.” Some polymers can be classified in two categories, with properties being greatly varied by varying molecular weight, end groups, processing, crosslinking, plasticizer, and so on. Nylon in its more crystalline from behaves as a fiber, whereas less crystalline forms are generally classified as plastics.
Many polymers can be treated to express more than one behavior. Thus, nylon-66 provides a good fibrous material when aligned and behaves as a plastic if it is not subjected to orientation. Polyesters also exhibit the same tendencies. Other materials, such as PVC and siloxanes, can be processed to act as plastics or elastomers.