MOLECULAR INTERACTIONS
The forces present in nature are often divided into primary forces (typically greater than 50 kcal/mol [200 kJ/mol] of interactions) and secondary forces (typically less than 10 kcal/mol [40 kJ/mol] of interactions). Primary bonding forces can be further subdivided into ionic (characterized by a lack of directional bonding; between atoms of largely differing electronegativities; not typically present within polymer backbones), metallic (the number of outer, valence electrons is too small to provide complete outer shells; often considered as charged atoms surrounded by a potentially fluid sea of electrons; lack of bonding direction; not typically found in polymers), and covalent (including coordinate and dative) bonding (which are the major means of bonding within polymers; directional).
The bonding lengths of primary bonds are usually about 0.90–2.0 A˚ (0.09–0.2 nm) with the carbon–carbon bond length being about 1.5–1.6 A˚ (0.15–0.16 nm). Atoms in individual polymer molecules are joined to each other by relatively strong covalent bonds. The bond energies of the carbon–carbon bonds are on the order of 80–90 kcal/mol (320–370 kJ/mol). Further more polymer molecules, like all other molecules, are attracted to each other (and for long-chain polymer chains even between segments of the same chain) by intermolecular, secondary forces. Secondary forces, frequently called van der Waals forces because they are the forces responsible for the van der Waals corrections to the ideal gas relationships, are of longer distance in interaction, in comparison to primary bond lengths, generally having significant interaction between 2.5 and 5 A˚ (0.25–0.5 nm).
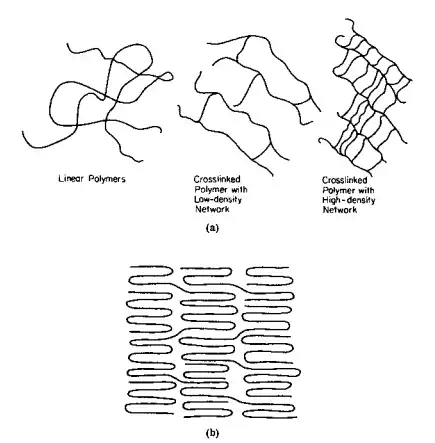
The force of these interactions is inversely proportional to some power of r, generally 2 or greater [force 1/(distance)r ] and thus is quite dependent on the distance between the interacting molecules. Thus, many physical properties of polymers are indeed quite dependent on both the conformation (arrangements related to rotation about single bonds) and configuration (arrangements related to the actual chemical bonding about a given atom), since both affect the proximity one chain can have relative to another. Thus, amorphous polypropylene is more flexible than crystalline polypropylene (compare linear polymers a (left) and b of Fig. 2.9). These intermolecular forces are also responsible for the increase in boiling points within a homologous series such as the alkanes, for the higher-than-expected boiling points.
of polar organic molecules such as alkyl chlorides, and for the abnormally high boiling points of alcohols, amines, and amides. While the forces responsible for these increases in boiling points are all called van der Waals forces, these are subclassified in accordance with their source and intensity. Secondary, intermolecular forces include London dispersion forces, induced permanent forces, and dipolar forces including hydrogen bonding. Nonpolar molecules such as ethane [H(CH2)2H] and polyethylene are attracted to each other by weak London or dispersion forces resulting from induced dipole–dipole interaction. The temporary or transient dipoles in ethane or along the polyethylene chain are due to instantaneous fluctuations in the density of the electron clouds.
The energy range of these forces is about 2 kcal/mol (8 kJ/mol) unit in nonpolar and polar polymers alike, and this force is independent of temperature. These London forces are typically the major forces present between chains in largely nonpolar polymers present in elastomers and soft plastics.
It is of interest to note that methane, ethane, and ethylene are all gases; hexane, octane, and nonane are all liquids (at room conditions); while polyethylene is a waxy solid. This trend is primarily due to both an increase in mass per molecule and to an increase in the London forces per molecule as the chain length increases. Assuming that the attraction between methylene or methyl units is 2 kcal/mol (8 kJ/mol), we calculate an interaction between methane to be 2 kcal/mol, hexane to be 12 kcal/mol, and for a mole of polyethylene chains of 1000 units to be 4000 kcal (16000 kJ).