STEREOCHEMISTRY OF POLYMERS
The terms “memory” and “to remember” are similar and used by polymer chemists in similar, but different, ways. The first use of the terms “memory” and “to remember” involves reversible changes in the polymer structure usually associated with stress–strain deformation of a rubber material where the dislodged, moved polymer segments are connected to one another through chemical and physical crosslinks, so that once the particular stress/strain is removed the polymer returns to its original, prestress–strain arrangement of the particular polymer segments. Thus, the polymer “remembers” its initial segmental arrangement and returns to it through the guiding of the crosslinks.
The second use involves nonreversible changes of polymer segments and whole chain movements also brought about through stress–strain actions. These changes include any synthetic chain and segmental orientations as well as postsynthesis changes including fabrications effects. These changes involve “permanent” changes in chain and segmental orientation and in some ways these changes represent the total history of the polymer materials from inception (synthesis) through the moment when a particular property or behavior is measured. These irreversible or nonreversible changes occur with both crosslinked and noncrosslinked materials and are largely responsible for the change in polymer property as the material moves from being synthesized, processed, fabricated, and used in what ever capacity it finds itself.
Thus, the polymeric material “remembers” its history with respect to changes and forces that influence chain and segmental chain changes. The ability of polymers to remember and have a memory are a direct consequence of their size. We can get an idea of the influence of size in looking at the series of methylene hydrocarbons as the number of carbon atoms increases. For low numbers of carbons.
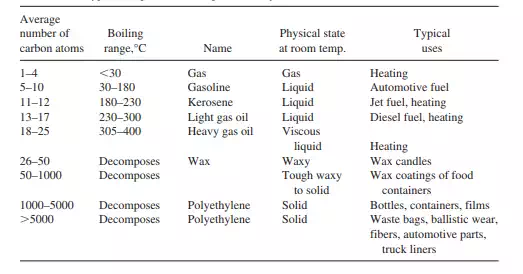
methane, ethane, propane, butane, the materials are gases at room temperature. For the next groupings (Table 2.1) the materials are liquids. The individual hydrocarbon chains are held together by dispersion forces that are a sum of the individual methylene and end group forces. There is a gradual increase in boiling point and total dispersion forces for the individual chains until the materials become waxy solids such as found in bees’ waxes and finally where the total dispersion forces are sufficient to be greater than individual carbon–carbon bond strengths, so that the chains decompose prior to their evaporation. Finally, the chain lengths are sufficient to give the tough and brittle solids we call polyethylene. It is interesting to note that these long chain straight chain hydrocarbons become very strong but brittle.
They
are crystalline—and as with most other crystalline materials, such as rocks and
diamonds, they are strong but brittle. Fortunately, synthetic polyethylene
contains both crystalline regions where the polymer chains are arranged in
ordered lines and regions where the chains are not arranged in ordered lines.
These latter arrangements are imposed on the polyethylene because of the
presence of branching in the linear polymer backbone. They are referred to as
amorphous regions and are responsible for allowing the polyethylene to have
some flexibility. Thus, many polymers contain both amorphous and crystalline
regions that provide both flexibility and strength. The polyethylene chains
described in Table 2.1 exhibit irreversible and, when appropriate crosslinking
is present, reversible memory. As a side note, low-molecular-weight
polyethylene with appreciable side branching has a melting range generally
below 100
C, whereas high-molecular-weight polyethylene with few branches has a melting
range approaching the theoretical value of about 145
C.
High-density
polyethylene (HDPE), formerly called low-pressure polyethylene [H(CH2CH2)nH],
like other alkanes [H(CH2)nH], may be used to illustrate a lot of polymer
structure. As in introductory organic chemistry, we can comprehend almost all
of the complex organic compounds if we understand the basic chemistry and
geometry. High-density polyethylene, like decane [H(CH2)10H] or paraffin
[H(CH2)50H], is a largely linear chain-like molecule consisting of catenated
carbon atoms bonded cova lently. The carbon atoms in all alkanes, including
HDPE, are joined at characteristic tetrahedral bond angles of approximately
109.5
. While decane consists of 10 methylene groups (CH2), HDPE may contain more
than 1000 of these groups. While we use the term normal straight chain or linear
for alkanes, we know that because of the characteristic bond angles the chains
are zigzag-shaped.
The distance between the carbon atoms is 1.54 angstroms (A˚ ) or 0.154 nanometers (nm). The apparent zigzag distance between carbon atoms in a chain of many carbon atoms is 1.26 A˚ , or 0.126 nm. Therefore, the length of an extended nonane chain would be 8 (1.26 A˚ ), or 10.08 A˚ , or 1.008 nm. Likewise, the length of an extended chain of HDPE having 1000 repeat ethylene units or structural elements [H(CH2CH2)1000H or H(CH2)2000H] would be 2520 A˚ or 252 nm. However, as shown by the magnified simulated structure in the diagram for HDPE (Fig. 2.1, top) because of rotation of the carbon–carbon
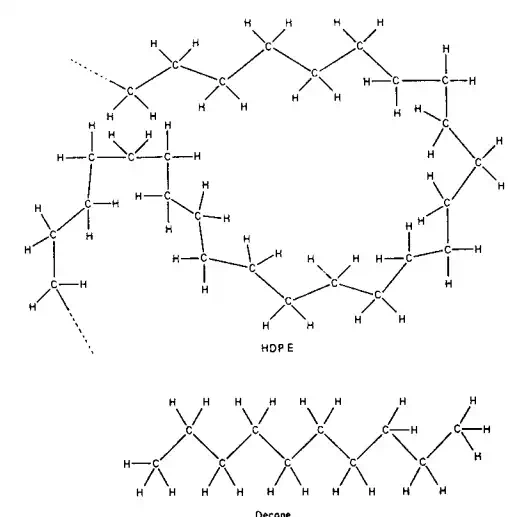
bonds, these chains are seldom extended to their full contour length but are present in many different shapes, or conformations.