Functional Groups
Different types of polymers are produced when the hydrogen atoms are substituted for other atoms or molecules, called functional groups.
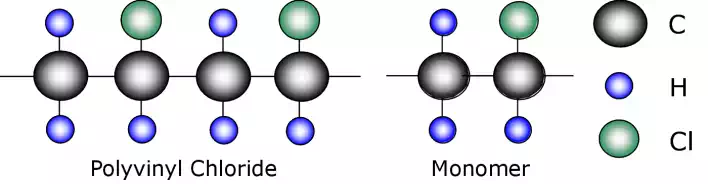
One of the most common engineering plastics is polyvinyl chloride, PVC. The structure of PVC consists of a chlorine, Cl, atom on every second monomer in the molecular chain.
When it is considered that PVC is a hard rigid plastic used for pipes and guttering while polyethylene is a soft flexible plastic used in bubble wrap then it is obvious that such a slight change can have a huge effect on polymer properties.
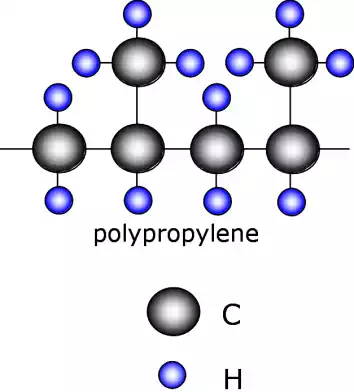
However, it is not only possible to substitute single atoms in the molecular structure. We can substitute whole molecules as well.
A typical example of this is polypropylene, PP, which substitutes a CH3 molecule on every second monomer.
Monomer Combinations
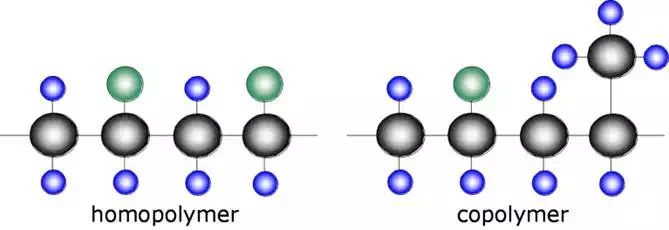
There is no reason for every monomer in a molecular chain to be the same. A polymer can be composed of a combination of different monomers.
A polymer composed of a single type of monomer is known as a homopolymer while a polymer composed of two or more different monomers is known as a copolymer. A copolymer is analogous to a metal alloy.