Properties
Ceramics are typically hard and brittle. While their strength in compression is very high, they are not suitable for loading in tension. Their brittle qualities mean that they fracture very easily. In compression, a crack is not easily propagated, but in tension the crack is free to grow.
Elastic Modulus
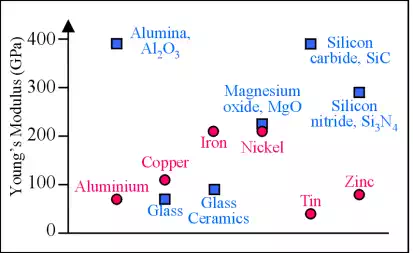
The elastic modulus (Young's Modulus) of ceramics is usually higher than for metals, because ceramics are bonded either covalently or ionically. This bonding is stronger than metallic bonding. Glasses have a lower modulus than other ceramics due to their non-uniform (amorphous) atomic structure.
Hardness

Ceramics and glasses are the hardest known materials. Many ceramics are often used as abrasives for this reason. They are hard because of their ordered structure, it is very difficult for dislocations to move through the atomic lattice.
Fracture Toughness
At room temperature, both ceramics and glasses will undergo fast fracture in a tensile test before any plastic deformation has occurred. Fast fracture is where the specimen breaks by the rapid initiation, growth and failure of a crack.
Ceramics have a fracture toughness about fifty times less than metals, even though their bonding forces are higher. Because of their susceptibility to cracking, consideration of the strength of ceramics requires consideration of their cracking behaviour. This quite often involves design and analysis with the use of fracture mechanics.
Ceramics are particularly susceptible to cracking because they usually contain many inherent small flaws or cracks. The tensile strength of a ceramic is determined by the length of the longest flaw.
It is because of the inherently low fracture toughness of most ceramics that they are usually loaded in compression. Ceramics have compressive strengths about ten times higher than their tensile strengths.
Strength
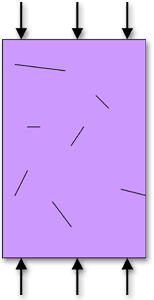
At room temperature, both ceramics and glasses undergo fast fracture in a tensile test before any plastic deformation occurs. However, ceramics have compressive strengths about ten times higher than their tensile strength. The tensile strength of ceramics and glasses is low because the existing flaws (internal or surface cracks) act as stress concentrators. However, flaws do not propagate under compression and, therefore, ceramics are usually used in applications where loads are compressive.
Thermal Shock
The effects of thermal shock can be seen when putting a hot ceramic dish into cold water and it shatters.
When you heat a material, it will expand slightly. Often running a stuck jar lid under the hot tap will loosen it. Conversely, cooling a material will result in a contraction.
When you have rapid changes in temperature, the material must be able to deform to compensate for the expansion or contraction. Metals and plastics usually are able to deform enough to compensate for this, but most ceramics (being too brittle) are not able to, and can fracture as a result. Glass is known to fracture upon a sudden change in temperature of more than 80°C. This is called thermal shock.
Melting Temperature
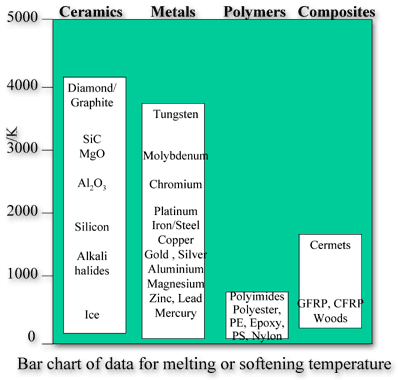
As a result of their high bond strengths, ceramics typically have very high melting temperatures, often much higher than metals and polymers. Most ceramics and glasses have a melting temperature above 2000°C. This means that they are often used in high temperature applications.
Creep
Similarly to metals, ceramics creep when they are hot. Creep is defined as slow plastic deformation at stress levels below the normal yield stress. Creep is very slow at room temperature for most materials. The difference between ceramics and metals is the temperature at which creep occurs. Creep occurs when the temperature is above about 0.4-0.5 Tm. Metals will therefore creep at relatively low temperatures - lead begins to creep at room temperature and aluminium alloys will begin to creep from about 250°C. Ceramics have melting temperatures much higher than metals and will only creep when they are exposed to stresses at much higher temperatures.