Structure and Form
Atomic Bonding
Ceramic and glass atomic structures are a network of either ionic or covalent bonds. Quite often they are a mixture of both. The individual structures are quite complex, so we will look briefly at the basic features in order that you can better understand their material properties.
Most ceramics have ionic bonding which leads to very high strength. These ceramics are typically a combination of a metal and a non-metal, e.g. sodium chloride NaCl or alumina Al2O3.
Some ceramics, however, have covalent bonding. These are either a combination of two non-metals, e.g. silica SiO2, or pure elements, e.g. diamond C.
Ionic Bonding
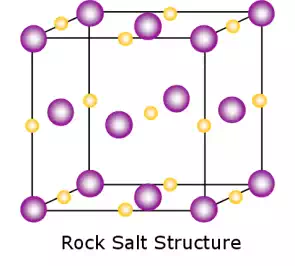
Ionic bonding is found in many ceramic structures such as NaCl, MgO and Al2O3. Atoms have unlike electrical charges, making them ions, which create an electrostatic attraction between atoms. This causes bonding between atoms. The ions pack into a regular arrangement. The nature of the ceramic depends on the size of the ion charges and the size of the ions.
For example, the structure shown left is the rock salt structure, such as that for NaCl.
Covalent Bonding
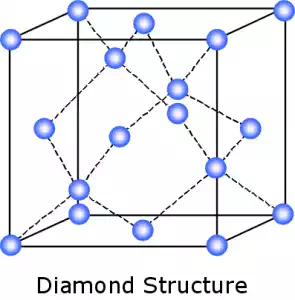
Covalent bonding is found in many ceramic structures such as SiC, BN and diamond. The atoms in these ceramics are arranged so that each pair of nearest neighbour atoms forms a chemical bond by sharing a pair of electrons.
The high energy of covalent bonds makes these ceramics very stable with regard to chemical and thermal changes.
Unlike ionic and metallic bonding, covalent bonding is directional and as a result, the atoms in many covalently bonded ceramics are arranged symmetrically to give a highly ordered structure. This gives them a very high hardness but also gives them a low toughness as they can be easily fractured along certain crystal planes.