Blending & Stabilisation
Blending and Alloying
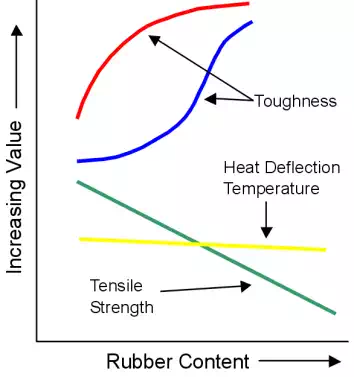
In blending, two immiscible (mutually insoluble) polymers are blended together to create a two-phase polymer. Generally, this will involve mixing an elastomer with a thermoplastic to create a plastic with small rubber regions that improve flexibility and toughness while maintaining a high level of tensile strength.
In alloying, two miscible polymers are blended together to create a single-phase polymer. In a similar manner to blending, the intention of alloying is to create a polymer with high toughness and flexibility as well as retained strength.
The graph shows the effect on ABS (Acrylonitrile-butadiene-styrene) properties when it is blended with increasing levels of rubber.
Stabilisation

Among the most costly problems to affect polymers is their susceptibility to attack by ultraviolet radiation. The energy imparted by an ultraviolet photon is enough to break a C-C bond. Quite often, stabilisers are added to absorb the radiation and prevent destruction of the polymer chains. Carbon black is the most common of these stabilisers, of which car tyres contain roughly 30wt%.
Polymers may also be degraded by oxygen in the atmosphere. Oxygen reacts with the molecular chains to create unwanted C–O-C cross- links. These cross-links raise the Tg and make the polymer brittle. Polymers are thus highly susceptible to ozone in the atmosphere.