Introduction to Polymers
Engineering materials commonly known as plastics, rubbers, and adhesives are all correctly referred to as polymers.
Over 15,000 different types of polymers are commercially available and are categorised into 20 different families.
Polymers are light, corrosion-resistant materials with low strength and stiffness. They are usually not suitable for high-temperature applications but are reasonably inexpensive and can be easily formed into a variety of shapes and designs.
Polymer Structure
Polymers are a range of materials that are composed of long chains of repeating molecules called monomers.
The long molecular chains intertwine to form complex compositional arrangements.
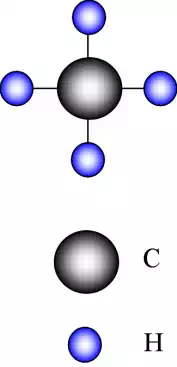
The backbone of these long molecules is the carbon, C, atom. From valence theory it is known that carbon has 4 electrons in its outer shell. It can therefore share a further 4 electrons to complete its atomic structure. In order to achieve this it must covalently bond with other atoms around it.
One of the more common atoms that carbon is likely to bond with is hydrogen, H.
It is known that hydrogen will share 1 electron to complete its outer shell, therefore carbon will bond with 4 hydrogen atoms.
However, polymers are supposed to be made up of long chain molecules. So, how are they made?
Polymer Chains
If two of the hydrogen atoms are removed and the carbon atom decides to bond with two other carbon atoms then it can be seen how long chains can be formed.
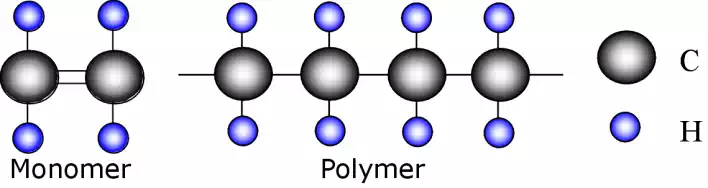
A carbon atom bonded to 2 hydrogen atoms can be considered a building block or unit of the polymeric chain. These units make up monomers, which are repeating units in the molecular chain structure.
A series of monomers bonded together to form a molecular chain is known as a polymer. The polymer shown is known as polyethylene.