SUPERCRITICAL WATER OXIDATION (SCWO) AND INDUSTRIAL SUPERCRITICAL WATER OXIDATION (ISCWO)
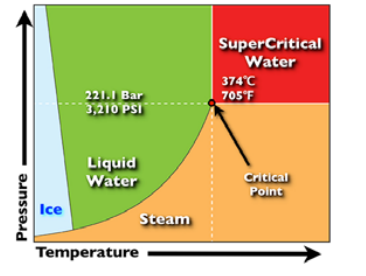
Both supercritical and subcritical water oxidation systems have been developed by a number of companies over the last 30 years and some have substantial commercial experience in destroying POPs such as PCB. The technologies share similar principles of destruction of organics using an oxidant agent such as hydrogen peroxide, oxygen, or nitrite. The term ‘supercritical’ refers to the state of water just prior to its phase change from liquid to gas under heat and pressure (e.g. 374 °C and 218 atmospheres). Subcritical water refers to the state of water just below its critical level (e.g. 370 °C and 262 atmospheres). In this state, organic materials can be rapidly oxidized and decomposed. For destruction of PCB typical reaction conditions are a temperature of 400 - 500 o C, pressure 25MPa with a reaction time of 1 - 5 minutes.
Supercritical systems are generally injected with the waste along with water and oxygen into a column—mixed, heated and compressed to the point of supercriticality. The system is totally enclosed. The properties of the water in this phase have elevated molecular kinematic energy that is highly reactive, and combined with oxygen, can oxidize and destroy organic waste. The outputs of the reaction are nitrogen, water, and carbon dioxide. The destruction of chlorinated POPs results in an output of elevated hydrochloric acid. The highly acidic environment this generates requires the structural equipment of the process vessels to be corrosion-resistant, such as titanium alloys in combination with anti-corrosive additives, such as sodium carbonate. The process is not suited to bulk solids, but can treat
Figure 37. Parameters for Supercritical water
aqueous wastes, oils, solvents, slurries, and solids with a diameter less than 200 μm. Early versions of the technology were prone to corrosion but his has been resolved with the use of corrosion-resistant materials.
As of 2013, there were 3 fully operational plants, 5 constructed, and 9 planned for construction. In the interim, many of these plants will have become operational. The longest established plant is operated in Japan by Japan Environmental Safety Corporation (JESCO) for PCB destruction, with a capacity of 2,000 kg of PCB per day . While costs can vary significantly due to the capacity and type of SCWO developed, a study by Aki et al. (1998) found that destruction of hazardous waste from the petrochemical industry could be achieved at significantly lower costs by implementing SCWO rather than by using incineration. Installation costs were 15% less expensive and running costs for SCWO were only around 10% of the costs of incineration of hazardous liquids. SCWO is now used extensively by the US military for destruction of hazardous wastes and chemical weapons, including mobile ship-based units.
Marrone, in summarizing a comprehensive review of the global state of SCWO, notes that “SCWO technology commercialization remains an area of great interest and activity.” The main advantages of SCWO are very low emissions, low costs, high destruction efficiency, and low associated resources (catalysts) for operation in remote locations. Studies have been conducted on plastic waste SCWO by researchers and industrial operators.
One company has developed the application to a higher degree of commercialization and can treat plastic waste. General Atomics has developed a relatively high throughput feed model designed for general industrial hazardous wastes, as well as non-hazardous waste. Their technology is referred to as Industrial Supercritical Water Oxidation or iSCWO. A GEFfunded project to treat large stockpiles of DDT waste in Kyrgyzstan is currently being implemented using the technology. While no stand-alone POPs plastics destruction facilities have been developed yet, the technology is capable of processing plastics. In addition, a number of SCWO plants have been operating to depolymerize plastics in Japan for two decades.
The General Atomics iSCWO operates with the following process: Air is pumped into the reactor vessel and pressurized to 3,200 psi and then heated to 650 o C. Water is pumped in and as the liquid flows into the reactor vessel pre-ignition is activated. Water is heated and pressurized above the thermodynamic critical point of 650 o C and 235 bar. When the stable critical point is reached, organic waste is mixed with quench water (and if required, sodium hydroxide). The supercritical conditions render organic materials, oxidation reactants, and oxidation products miscible in water and they are oxidised and destroyed. The remaining liquid is then discharged through a pressure let-down to atmospheric conditions.

The liquid and gas waste products from the process consist of carbon dioxide, water, and depending on the waste feed, salts and metallic oxides. Steam is vented to the atmosphere. There are no particulates released or pollution abatement filters required. Clean water is produced requiring no pre-treatment to dispose to sewer (elevated salinity and metal oxides limit the use of the effluent water).
