Effects due to Climate, Temperature, Chemical, Corrosion on Concrete
Effects due Climate
The lack of durability of concrete on account of freezing and thawing action of frost is not of great importance to Indian conditions. But it is of greatest considerations in most part of the world.
The most severe climatic attack on concrete occurs, when concrete containing moisture is subjected to cycle of freezing and thawing. The capillary pores in the cement paste are of such a size that water in them will freeze, when the ambient temperature is below 0degree C.
The gel pores are so small that water in them does freeze at normal winter temperatures. As water, when freezing expands by 9% of its volume, excess water in the capillaries has to move. Since the cement paste is relatively impermeable high pressures are necessary to move the excess water even over quite small distances. For normal strength concrete, it has been found that movement of the order of 0.2mm is sufficient to require pressures which approach the tensile strength of the paste.
Concrete can be protected from freeze-thaw damage by the entrainment of the appropriate quantities of air distributed through the cement paste, with spacing between bubbles of not more than about 0.4mm. The air bubbles must remain partially empty, so that they can accommodate the excess water moved to them. This will generally be the case, since the bubbles constitute the coarsest pore system, and are therefore the first to, most moisture as the concrete dries. Fully saturated concrete, if permanently submerged, will not need protection against freezing, but concrete which has been saturated and is exposed to freezing as for example in the tidal range, may not be effectively protected by air entrainment.
For effective protection, an air entraining agent must be added to the mix, to entrain the appropriate amount of air, and to induce a bubble system, with an appropriate spacing. When AEA is used, it is only the amount of air entrained which can be measured in the wet concrete. The amount of air required is between 4-8%, depending on the maximum size of aggregate. Air is entrained during the mixing action, even when no AEA is added. The effect of AEA is to stabilize the air bubbles in the form desired.
More air is entrained with a larger dose of AEA but the effect is not linear and with most agents levels off larger doses. For mixes with higher slump, more air is entrained. It is difficult to entrain air is very stiff mixes; the grading and nature of the particles in the fine aggregates have a very marked effect, on the amount of air entrained. It has been shown that the sand is the most important single factor in air entrainment.
It has been suggested that if concrete can be so dense, that there are no inter-connected capillary pores, and then resistances to freeze- thaw deterioration will exist without the need for air entrainment.
The use of high cement content and low w/c ratio will lead in this direction as will the introduction of silica flume, but there is yet firm evidence to show that, it would be wise to dispense with air-entrainment, if freeze-thaw resistance is wanted.
Effects due to temperature
Temperatures of concrete, other than special refractory concrete, have to be kept below 300degrre
C. Heat may affect concrete as result of:
· The removal of evaporable water
· The removable of combined water
· Alteration of cement paste
· Disruption from disparity of expansion and resulting thermal stress
· Alteration of aggregate
· Change of the bond between aggregate and paste
Effects due to Chemical
Some of the factors, which increases the vulnerability of concrete to external chemical attack:
· High porosity, and hence high permeability
· Improper choice of cement type for the conditions of exposure
· Inadequate curing prior to exposure
· Exposure to alternate cycles of wetting and drying and to a lesser extent heating and cooling, with allowance for the fact that higher temperature increase reactivity
· Increased fluid velocities
· Expansive reactions of any sort which may cause cracking and any other physical phenomena, which lead to greater exposure of reactant surfaces
· Suction forces
· Unsatisfactory choice of shape and surface to volume ratios of concrete section
Effects due to Corrosion
Corrosion is defined as the process of deterioration (or destruction) and consequent loss of a solid metallic material, through an unwanted (or unintentional) chemical or electro-chemical attack by its environment, starting at its surface, is called Corrosion. Thus corrosion is a process of 'reverse
of extraction of metals'.
Corrosion Mechanism - Wet or Electro-Chemical Corrosion
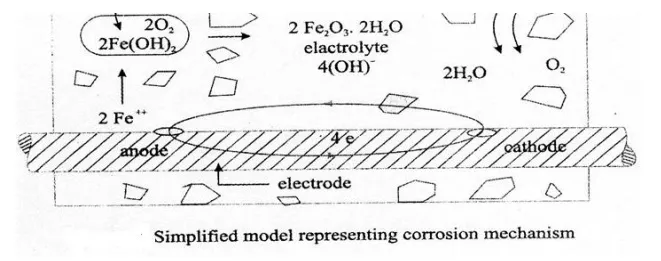
Corrosion of steel concrete is an electro-chemical process. When there is a difference in electrical potential, along the reinforcement in concrete, an electro-chemical cell is set up. In the steel, one part becomes anode (an electrode with a +ve charge) and other part becomes cathode, (an electrode with a -ve charge) connected by electrolyte in the form of pore water, in the hardened cement paste. The +vely charged ferrous ions Fe+ at the anode pass into solution, while the -vely charged free electrons -pass through the steel into cathode, where they are absorbed by the constituents of the electrolyte, and combine with water and oxygen to form hydroxyl ions (OH).
These travel through the electrolyte and combine with the ferrous ions to form ferric hydroxide, which is converted by further oxidation to rust.
The reactions are described below:
Anodic Reactions:
Fe - - > Fe+++ 2e-
Fe+++ 2(OH)2 - - > Fe(OH)2
Fe(OH)2.2H2O +O2 -- > 4Fe(OH)3
Cathodic Reaction
4e- +O2+H2O -- - > 4(OH)

It can be noted that no corrosion takes place if the concrete is dry probably below relative humidity of 60%, because enough water is not there to promote corrosion. it can also be noted that corrosion does not take place, id concrete is fully immersed in water, because diffusion of oxygen does not take place into the concrete. Probably the optimum relative humidity for corrosion is 70-80%
The products of corrosion occupy a volume as much as 6 times the original volume of steel, depending upon the oxidation state. Figure below shows the increase in volume of steel, depending upon the oxidation state.
it may be pointed out that though the 2 reactions Fe2 and OH originate iron the anode and cathode respectively, their combination occurs more commonly at the cathode, because the Sn. alert Fe2+ ions diffuse more rapidly than the larger OH ions. So, corrosion occurs at the anode, but rust is deposited at or near the cathode.
Increase the oxygen content has 2 effects:
(i) It forces the cathode reaction to the right, producing more OH- ions and
(ii) It removes more electrons and therefore, accelerates the corrosion at the anode.
Each of these effects, in turn, supplies more reactants for the forming reaction. Obviously, presence of oxygen greatly accelerates both corrosion and rust formation, with the corrosion occurring the entire anode, but the rust forming at the cathode.