Prevention of Corrosion Explained: A Simple Electroplating
Experiment
A large quantity of iron and
steel is damaged because of corrosion, causing huge losses that may have been
minimized by the adoption of appropriate preventive measures. Corrosion
products from oxidation do not adhere to the metal surface, and the resultant
pitting deteriorates the structure.
What Is Corrosion

Corrosion is a natural event
that causes the weakening ofa material,
usually a metal, or its characteristics due to reactions with the environment.
Some environments are more
suitable for the chemical combination of metals with elements to create
compounds and come back to their low energy levels.
Corrosion is a serious
condition of the substance that may produce massive damage to the product,
including bridges, buildings, water systems, and home appliances, unless
suitable prevention and control techniques are applied.
The Chemical Reaction In Corrosion
A substance disintegrates into
atoms because of the chemical reactions with the water and oxygen in the
environment, resulting into an electron loss of the material.
If an electrical circuit is
completed, the metal atoms become positively charged ions, causing pitting or
the development of a crack.
The rate of pitting corrosion
is greater in portions where welding operations have caused micro structural
transformations. Localized corrosion may initiate fatigue that can intensify by
the action with corrosive agents like seawater.
In an electrochemical
corrosion, the strength of iron is reduced due to the oxidation of its atoms
that is called rusting, by which oxides are formed.
Rusting Of Iron

The most significant category
of corrosionis the rusting of iron, which
produces iron oxides due to the reaction of iron with oxygen in an environment
of humid air or water.
Metal corrosion will also
occur by a chemical reaction with gaseous substances like acid vapors, ammonia gas, and gases containing sulpher.
Corrosion particularly
signifies the process that is related to the weakening or degradation of the
metal parts, and the processes are generally electrochemical in nature. Rust
formed is brittle and prominent as a reddish crust on the exposed fresh iron
surface.
Formation of rust can be
minimized by the exclusion of the air and water from the surface of the iron by
the application of paint, oil, grease, or a shielding coat of another metal
such as chromium, zinc, or nickel. Stainless steels do not corrode because of
the addition of protective coatings of nickel or chromium that form a rigid coating
to withstand additional attack.
Rust Prevention By Galvanization

Rust prevention is important
to avoid damage to expensive equipment and appliances. Several techniques are
being employed for this purpose.
Galvanization is a
metallurgical process in which a zinc coating is applied on steel or iron to
avoid rust, since the corrosion resistance of zinc is superior to those of
steel and iron.
Coatings of zinc achieve
corrosion prevention of the protected metal by the formation of a physical
obstruction, and by functioning as an anode if this obstruction is destroyed.
On exposure of zinc to the
atmosphere, zinc oxide is formed by the reaction of zinc with oxygen that
further reacts with molecules of water in the air to form zinc hydroxide.
Reaction of zinc hydroxide
with carbon dioxide in the atmosphere creates a thin and insoluble layer of
zinc carbonate that prevents further corrosion. Preservation of iron and steel
by the process of galvanization is preferred because it is economical and
simple in application.
What is Electroplating
Electroplating is another
method through which iron or steel can be protected and prevented from rusting
and corroding. Here, the metal to be protected is coated by a thin layer of
another metal having non-rusting properties by reducing it.
Normally, the metals involved
form the electrodes, which are processed inside an electrolyte by passing
electric current (DC) across the electrodes, through the electrolyte.
In this process the electrode
which is connected to the negative of the supply gradually gets covered with
the metal of the electrode connected to the positive of the electric supply
which slowly disintegrates or reduces and becomes attached over the other
electrode.
The electrode connected to the
negative is the one which is being electroplated for the required protections.
The above process can be
explained and witnessed through a small experiment.
You will need the following
materials for the experiment:
One iron nail
One copper rod
Water
Copper sulphate crystals
A 9 Volt Battery
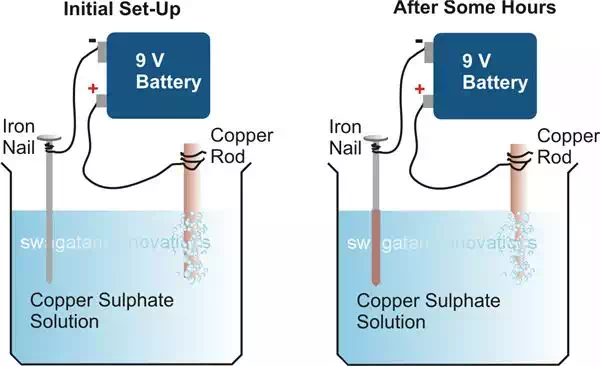
Procedure:
Take a vessel and fill it with
water, add a teaspoon full of copper sulphate crystals in the water and mix it
thoroughly.
Copper sulphate not only helps
to enhance electricity conduction through water, but also directly participates
in the process by extracting copper from the copper rod and attaching it over
the exterior of the nail.
Arrange the nail and the
copper rod such that some part of them are immersed in the solution and are
placed rigidly and vertically, as shown in the diagram.
Make sure that the metals do
not touch each other.
Obviously, here the iron nail
is the component we want to electroplate or cover with some kind of layer. So
the nail has to become the cathode terminal and the copper rod can be selected
as the anode terminal.
Connect them appropriately to
the battery poles, as explained above.
The electroplating process
will be instantly initiated and after a few hours you should be able to find
the dipped portion nail completely covered with copper deposits, received from
the copper rod which in the course can be seen to have become much thinner
(eroded) over the immersed area.
Electroplating, an Experiment