Nuclear fission
Nuclear fission, subdivision of a heavy atomic nucleus, such as that of uranium or plutonium, into two fragments of roughly equal mass. The process is accompanied by the release of a large amount of energy. In nuclear fission the nucleus of an atom breaks up into two lighter nuclei. The process may take place spontaneously in some cases or may be induced by the excitation of the nucleus with a variety of particles (e.g., neutrons, protons, deuterons, or alpha particles) or with electromagnetic radiation in the form of gamma rays. In the fission process, a large quantity of energy is released, radioactive products are formed, and several neutrons are emitted. These neutrons can induce fission in a nearby nucleus of fissionable material and release more neutrons that can repeat the sequence, causing a chain reaction in which a large number of nuclei undergo fission and an enormous amount of energy is released. If controlled in a nuclear reactor, such a chain reaction can provide power for society’s benefit. If uncontrolled, as in the case of the so-called atomic bomb, it can lead to an explosion of awesome destructive force.
The discovery of nuclear fission has opened a new era—the “Atomic Age.” The potential of nuclear fission for good or evil and the risk/benefit ratio of its applications have not only provided the basis of many sociological, political, economic, and scientific advances but grave concerns as well. Even from a purely scientific perspective, the process of nuclear fission has given rise to many puzzles and complexities, and a complete theoretical explanation is still not at hand.
History Of Fission Research And Technology
The term fission was first used by the German physicists Lise Meitnerand Otto Frisch in 1939 to describe the disintegration of a heavy nucleus into two lighter nuclei of approximately equal size. The conclusion that such an unusual nuclear reaction can in fact occur was the culmination of a truly dramatic episode in the history of science, and it set in motion an extremely intense and productive period of investigation.
The story of the discovery of nuclear fission actually began with the discovery of the neutron in 1932 by James Chadwick in England. Shortly thereafter Enrico Fermi and his associates in Italy undertook an extensive investigation of the nuclear reactions produced by the bombardment of various elements with this uncharged particle. In particular, these workers observed (1934) that at least four different radioactive species resulted from the bombardment of uranium with slow neutrons. These newly discovered species emitted beta particles and were thought to be isotopes of unstable “transuranium elements” of atomic numbers 93, 94, and perhaps higher. There was, of course, intense interest in examining the properties of these elements, and many radiochemists participated in the studies. The results of these investigations, however, were extremely perplexing, and confusion persisted until 1939 when Otto Hahn and Fritz Strassmann in Germany, following a clue provided by Irène Joliot-Curie and Pavle Savić in France (1938), proved definitely that the so-called transuranic elements were in fact radioisotopes of barium, lanthanum, and other elements in the middle of the periodic table.
That lighter elements could be formed by bombarding heavy nuclei with neutrons had been suggested earlier (notably by the German chemist Ida Noddack in 1934), but the idea was not given serious consideration because it entailed such a broad departure from the accepted views of nuclear physics and was unsupported by clear chemical evidence. Armed with the unequivocal results of Hahn and Strassmann, however, Meitner and Frisch invoked the recently formulated liquid-drop model of the nucleus to give a qualitative theoretical interpretation of the fission process and called attention to the large energy release that should accompany it. There was almost immediate confirmation of this reaction in dozens of laboratories throughout the world, and within a year more than 100 papers describing most of the important features of the process were published. These experiments confirmed the formation of extremely energetic heavy particles and extended the chemical identification of the products. The chemical evidence that was so vital in leading Hahn and Strassmann to the discovery of nuclear fission was obtained by the application of carrier and tracer techniques. Since invisible amounts of the radioactive species were formed, their chemical identity had to be deduced from the manner in which they followed known carrier elements, present in macroscopic quantity, through various chemical operations. Known radioactive species were also added as tracers and their behaviour was compared with that of the unknown species to aid in the identification of the latter. Over the years, these radiochemical techniques have been used to isolate and identify some 34 elements from zinc (atomic number 30) to gadolinium (atomic number 64) that are formed as fission products. The wide range of radioactivities produced in fission makes this reaction a rich source of tracers for chemical, biologic, and industrial use.
Although the early experiments involved the fission of ordinary uranium with slow neutrons, it was rapidly established that the rare isotope uranium-235 was responsible for this phenomenon. The more abundant isotope uranium-238 could be made to undergo fission only by fast neutrons with energy exceeding 1 MeV. The nuclei of other heavy elements, such as thorium and protactinium, also were shown to be fissionable with fast neutrons; and other particles, such as fast protons, deuterons, and alphas, along with gamma rays, proved to be effective in inducing the reaction. In 1939, Frédéric Joliot-Curie, Hans von Halban, and Lew Kowarski found that several neutrons were emitted in the fission of uranium-235, and this discovery led to the possibility of a self-sustaining chain reaction. Fermi and his coworkers recognized the enormous potential of such a reaction if it could be controlled. On Dec. 2, 1942, they succeeded in doing so, operating the world’s first nuclear reactor. Known as a “pile,” this device consisted of an array of uranium and graphite blocks and was built on the campus of the University of Chicago. The secret Manhattan Project, established not long after the United States entered World War II, developed the atomic bomb. Once the war had ended, efforts were made to develop new reactor types for large-scale power generation, giving birth to the nuclear powerindustry.
Fundamentals Of The Fission Process
Structure and stability of nuclear matter
The fission process may be best understood through a consideration of the structure and stability of nuclear matter. Nuclei consist of nucleons(neutrons and protons), the total number of which is equal to the mass number of the nucleus. The actual mass of a nucleus is always less than the sum of the masses of the free neutrons and protons that constitute it, the difference being the mass equivalent of the energy of formation of the nucleus from its constituents. The conversion of mass to energy follows Einstein’s equation, E = mc2, where E is the energy equivalent of a mass, m, and c is the velocity of light. This difference is known as the mass defect and is a measure of the total binding energy(and, hence, the stability) of the nucleus. This binding energy is released during the formation of a nucleus from its constituentnucleons and would have to be supplied to the nucleus to decompose it into its individual nucleon components.
A curve illustrating the average binding energy per nucleon as a function of the nuclear mass number is shown in Figure 1. The largest binding energy (highest stability) occurs near mass number 56—the mass region of the element iron. Figure 1 indicates that any nucleus heavier than mass number 56 would become a more stable system by breaking into lighter nuclei of higher binding energy, the difference in binding energy being released in the process. (It should be noted that nuclei lighter than mass number 56 can gain in stability by fusing to produce a heavier nucleus of greater mass defect—again, with the release of the energy equivalent of the mass difference. It is the fusion of the lightest nuclei that provides the energy released by the Sun and constitutes the basis of the hydrogen, or fusion, bomb. Efforts to harness fusion reaction for power production have been actively pursued. [See nuclear fusion.])
On the basis of energy considerations alone, Figure 1 would indicate that all matter should seek its most stable configuration, becoming nuclei of mass number near 56. However, this does not happen, because barriers to such a spontaneous conversion are provided by other factors. A good qualitative understanding of the nucleus is achieved by treating it as analogous to a uniformly charged liquid drop. The strong attractive nuclear force between pairs of nucleons is of short range and acts only between the closest neighbours. Since nucleons near the surface of the drop have fewer close neighbours than those in the interior, a surface tension is developed, and the nuclear drop assumes a spherical shape in order to minimize this surface energy. (The smallest surface area enclosing a given volume is provided by a sphere.) The protons in the nucleus exert a long-range repulsive (Coulomb) force on each other because of their positive charge. As the number of nucleons in a nucleus increases beyond about 40, the number of protons must be diluted with an excess of neutrons to maintain relative stability.
If the nucleus is excited by some stimulus and begins to oscillate (i.e., deform from its spherical shape), the surface forces will increase and tend to restore it to a sphere, where the surface tension is at a minimum. On the other hand, the Coulomb repulsion decreases as the drop deforms and the protons are positioned farther apart. These opposing tendencies set up a barrier in the potential energy of the system, as indicated in Figure 2.
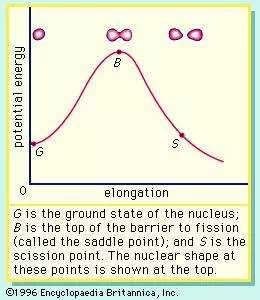
Figure 2: The potential energy as a function of elongation of a fissioning nucleus. G is the ground state of the nucleus; B is the top of the barrier to fission (called the saddle point); and S is the scission point. The nuclear shape at these points is shown at the top.Encyclopædia Britannica, Inc.
The curve in Figure 2 rises initially with elongation, since the strong, short-range nuclear force that gives rise to the surface tension increases. The Coulomb repulsion between protons decreases faster with elongation than the surface tension increases, and the two are in balance at point B, which represents the height of the barrier to fission. (This point is called the “saddle point” because, in a three-dimensional view of the potential energy surface, the shape of the pass over the barrier resembles a saddle.) Beyond point B, the Coulomb repulsion between the protons drives the nucleus into further elongation until at some point, S (the scission point), the nucleus breaks in two. Qualitatively, at least, the fission process is thus seen to be a consequence of the Coulomb repulsion between protons. Further discussion of the potential energy in fission is provided below.
Induced fission
The height and shape of the fission barrier are dependent on the particular nucleus being considered. Fission can be induced by exciting the nucleus to an energy equal to or greater than that of the barrier. This can be done by gamma-ray excitation (photofission) or through excitation of the nucleus by the capture of a neutron, proton, or other particle (particle-induced fission). The binding energy of a particular nucleon to a nucleus will depend on—in addition to the factors considered above—the odd–even character of the nucleus. Thus, if a neutron is added to a nucleus having an odd number of neutrons, an even number of neutrons will result, and the binding energy will be greater than for the addition of a neutron that makes the total number of neutrons odd. This “pairing energy” accounts in part for the difference in behaviour of nuclides in which fission can be induced with slow (low-energy) neutrons and those that require fast (higher-energy) neutrons. Although the heavy elements are unstable with respect to fission, the reaction takes place to an appreciable extent only if sufficient energy of activation is available to surmount the fission barrier. Most nuclei that are fissionable with slow neutrons contain an odd number of neutrons (e.g., uranium-233, uranium-235, or plutonium-239), whereas most of those requiring fast neutrons (e.g., thorium-232 or uranium-238) have an even number. The addition of a neutron in the former case liberates sufficient binding energy to induce fission. In the latter case, the binding energy is less and may be insufficient to surmount the barrier and induce fission. Additional energy must then be supplied in the form of the kinetic energy of the incident neutron. (In the case of thorium-232 or uranium-238, a neutron having about 1 MeV of kinetic energy is required.)
Spontaneous fission
The laws of quantum mechanics deal with the probability of a system such as a nucleus or an atom being in any of its possible states or configurations at any given time. A fissionable system (uranium-238, for example) in its ground state (i.e., at its lowest excitation energy and with an elongation small enough that it is confined inside the fission barrier) has a small but finite probability of being in the energetically favoured configuration of two fission fragments. In effect, when this occurs, the system has penetrated the barrier by the process of quantum mechanical tunneling. This process is called spontaneous fission because it does not involve any outside influences. In the case of uranium-238, the process has a very low probability, requiring more than 1015 years for half of the material to be transformed (its so-called half-life) by this reaction. On the other hand, the probability for spontaneous fission increases dramatically for the heaviest nuclides known and becomes the dominant mode of decay for some—those having half-lives of only fractions of a second. In fact, spontaneous fission becomes the limiting factor that may prevent the formation of still heavier (super-heavy) nuclei.
The Stages Of Fission
A pictorial representation of the sequence of events in the fission of a heavy nucleus is given in Figure 3. The approximate time elapse between stages of the process is indicated at the bottom of the Figure.
·
·
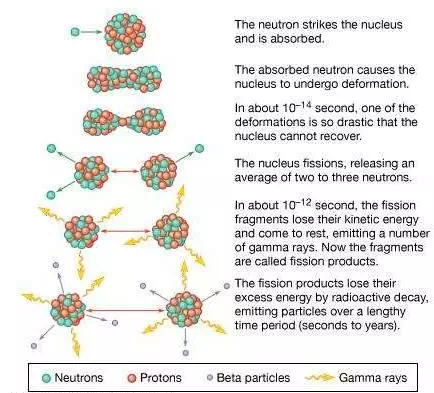
nuclear fissionSequence of events in the fission of a uranium nucleus by a neutron.
The Phenomenology Of Fission
When a heavy nucleus undergoes fission, a variety of fragment pairs may be formed, depending on the distribution of neutrons and protons between the fragments. This leads to probability distributionof both mass and nuclear charge for the fragments. The probability of formation of a particular fragment is called its fission yield and is expressed as the percentage of fissions leading to it. The separated fragments experience a large Coulomb repulsion due to their nuclear charges, and they recoil from each other with kinetic energies determined by the fragment charges and the distance between the charge centres at the time of scission. Variations in these parameters lead to a distribution of kinetic energies, even for the same mass split.
The initial velocities of the recoiling fragments are too fast for the outer (atomic) electrons of the fissioning atom to keep pace, and many of them are stripped away. Thus, the nuclear charge of the fragment is not fully neutralized by the atomic electrons, and the fission fragments fly apart as highly charged atoms. As the nucleus of the fragment adjusts from its deformed shape to a more stable configuration, the deformation energy (i.e., the energy required to deform it) is recovered and converted into internal excitation energy, and neutrons and prompt gamma rays (an energetic form of electromagnetic radiation given off nearly coincident with the fission event) may be evaporated from the moving fragment. The fast-moving, highly charged atom collides with the atoms of the medium through which it is moving, and its kinetic energy is transferred to ionization and heating of the medium as it slows down and comes to rest. The range of fission fragments in air is only a few centimetres.
During the slowing-down process, the charged atom picks up electrons from the medium and becomes neutral by the time it stops. At this stage in the sequence of events, the atom produced is called a fission product to distinguish it from the initial fission fragment formed at scission. Since a few neutrons may have been lost in the transition from fission fragment to fission product, the two may not have the same mass number. The fission product is still not a stable species but is radioactive, and it finally reaches stability by undergoing a series of beta decays, which may vary over a time scale of fractions of a second to many years. The beta emission consists of electrons and antineutrinos, often accompanied by gamma rays and X-rays.
The distributions in mass, charge, and kinetic energy of the fragments have been found to be dependent on the fissioning species as well as on the excitation energy at which the fission act occurs. Many other aspects of fission have been observed, adding to the extensive phenomenology of the process and providing an intriguing set of problems for interpretation. These include the systematics of fission cross sections (a measure of the probability for fission to occur); the variation of the number of prompt neutrons (see below) emitted as a function of the fissioning species and the particular fragment mass split; the angular distribution of the fragments with respect to the direction of the beam of particles inducing fission; the systematics of spontaneous fission half-lives; the occurrence of spontaneous fission isomers (excited states of the nucleus); the emission of light particles (hydrogen-3, helium-3, helium-4, etc.) in small but significant numbers in some fission events; the presence of delayed neutron emitters among the fission products; the time scale on which the various stages of the process take place; and the distribution of the energy release in fission among the particles and radiations produced. A detailed discussion of all of these facets of fission and how the data were obtained is not possible here, but a few of them are treated to provide some insight into this field of study and a taste of its fascination.
Fission fragment mass distributions
The distribution of the fragment masses formed in fission is one of the most striking features of the process. It is dependent on the mass of the fissioning nucleus and the excitation energy at which the fission occurs. At low excitation energy, the fission of such nuclides as uranium-235 or plutonium-239 is asymmetric; i.e., the fragments are formed in a two-humped probability (or yield) distribution favouring an unequal division in mass. This is illustrated in Figure 4. As will be noted, the light group of fragment masses shifts to higher mass numbers as the mass of the fissioning nucleus increases, whereas the position of the heavy group remains nearly stationary. As the excitation energy of the fission increases, the probability for a symmetric mass split increases, while that for asymmetric division decreases. Thus, the valley between the two peaks increases in probability (yield of formation), and at high excitations the mass distribution becomes single-humped, with the maximum yield at symmetry (see Figure 5). Radium isotopes show interesting triple-humped mass distributions, and nuclides lighter than radium show a single-humped, symmetric mass distribution. (These nuclides, however, require a relatively high activation energy to undergo fission.) For very heavy nuclei in the region of fermium-260, the mass-yield curve becomes symmetric (single-humped) even for spontaneous fission, and the kinetic energies of the fragments are unusually high. An understanding of these mass distributions has been one of the major puzzles of fission, and a complete theoretical interpretation is still lacking, albeit much progress has been made (see below).

Figure 5: Mass distribution dependence on the energy excitation in the fission of uranium-235. At still higher energies, the curve becomes single-humped, with a maximum yield for symmetric mass splits
Fission decay chains and charge distribution
In order to maintain stability, the neutron-to-proton (n/p) ratio in nuclei must increase with increasing proton number. The ratio remains at unity up to the element calcium, with 20 protons. It then gradually increases until it reaches a value of about 1.5 for the heaviest elements. When a heavy nucleus fissions, a few neutrons are emitted; however, this still leaves too high an n/p ratio in the fission fragments to be consistent with stability for them. They undergo radioactive decay and reach stability by successive conversions of neutrons to protons with the emission of a negative electron (called a beta particle, β-) and an antineutrino. The mass number of the nucleus remains the same, but the nuclear charge (atomic number) increases by one, and a new element is formed for each such conversion. The successive beta decays constitute an isobaric, fission-product decay chain for each mass number. The half-lives for the decay of the radioactive species generally increase as they approach the stable isobar of the chain. (Species of the same element characterized by the same nuclear charge, Z [number of protons], but differing in their number of neutrons [and therefore in mass number A] are called isotopes. Species that have the same mass number, A, but differ in Z are known as isobars.)
For a typical mass split in the neutron-induced fission of uranium-235, the complementary fission-product masses of 93 and 141 may be formed following the emission of two neutrons from the initial fragments. The division of charge (i.e., protons) between the fragments represents an important parameter in the fission process. Thus, for the mass numbers 93 and 141, the following isobaric fission-product decay chains would be formed (the half-lives for the beta-decay processes are indicated above the arrows):
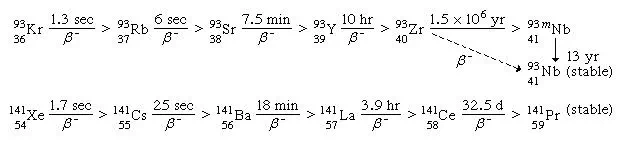
(The left subscript on the element symbol denotes Z, while the superscript denotes A.) The 92 protons of the uranium nucleus must be conserved, and complementary fission-product pairs—such as krypton-36 with barium-56, rubidium-37 with cesium-55, or strontium-38 with xenon-54—would be possible.
The percentage of fissions in which a particular nuclide is formed as a primary fission product (i.e., as the direct descendant of an initial fragment following its de-excitation) is called the independent yield of that product. The total yield for any nuclide in the isobaric decay chain is the sum of its independent yield and the independent yields of all of its precursors in the chain. The total yield for the entire chain is called the cumulative yield for that mass number.
Extensive radiochemical investigations have suggested that the most probable charge division is one that is displaced from stability about the same distance in both chains. This empirical observation is called the equal charge displacement (ECD) hypothesis, and it has been confirmed by several physical measurements. In the above example the ECD would predict the most probable charges at about rubidium-37 and cesium-55. A strong shell effect modifies the ECD expectations for fragments having 50 protons. The dispersion of the charge formation probability about the most probable charge (Zp) is rather narrow and approximately Gaussian in shape and is nearly independent of the mass split as well as of the fissioning species. The most probable charge for an isobaric chain is a useful concept in the description of the charge dispersion, and it need not have an integralvalue. As the energy of fission increases, the charge division tends toward maintaining the n/p ratio in the fragments the same as that in the fissioning nucleus. This is referred to as an unchanged charge distribution.
Prompt neutrons in fission
The average number of neutrons emitted per fission (represented by the symbol v̄) varies with the fissioning nucleus. It is about 2.0 for the spontaneous fission of uranium-238 and 4.0 for that of fermium-257. In the thermal-neutron induced fission of uranium-235, v̄= 2.4. The actual number of neutrons emitted, however, varies with each fission event, depending on the mass split. Although there is still controversy regarding the number of neutrons emitted at the instant of scission, it is generally agreed that most of the neutrons are given off by the recoiling fission fragments soon after scission occurs. The number of neutrons emitted from each fragment depends on the amount of energy the fragment possesses. The energy can be in the form of internal excitation (heat) energy or stored as energy of deformation of the fragment to be released when the fragment returns to its stable equilibrium shape.
Delayed neutrons in fission
A few of the fission products have beta-decay energies that exceed the binding energy of a neutron in the daughter nucleus. This is likely to happen when the daughter nucleus contains one or two neutrons more than a closed shell of 50 or 82 neutrons, since these “extra” neutrons are more loosely bound. The beta decay of the precursor may take place to an excited state of the daughter from which a neutron is emitted. The neutron emission is “delayed” by the beta-decay half-life of the precursor. Six such delayed neutron emitters have been identified, with half-lives varying from about 0.5 to 56 seconds. The yield of the delayed neutrons is only about 1 percent of that of the prompt neutrons, but they are very important for the control of the chain reaction in a nuclear reactor.
Energy release in fission
The total energy release in a fission event may be calculated from the difference in the rest masses of the reactants (e.g., 235U + n) and the final stable products (e.g., 93Nb + 141Pr + 2n). The energy equivalent of this mass difference is given by the Einstein relation, E = mc2. The total energy release depends on the mass split, but a typical fission event would have the total energy release distributed approximately as follows for the major components in the thermal neutron-induced fission of uranium-235:

(The energy release from the capture of the prompt neutrons depends on how they are finally stopped, and some will escape the core of a nuclear reactor.)
This energy is released on a time scale of about 10-12 second and is called the prompt energy release. It is largely converted to heat within an operating reactor and is used for power generation. Also, there is a delayed release of energy from the radioactive decay of the fission products varying in half-life from fractions of a second to many years. The shorter-lived species decay in the reactor, and their energy adds to the heat generated; however, the longer-lived species remain radioactive and pose a problem in the handling and disposition of the reactor fuel elements when they need to be replaced. The antineutrinos that accompany the beta decay of the fission products are unreactive, and their kinetic energy (about 10 MeV per fission) is not recovered. Overall, about 200 MeV of energy per fission may be recovered for power applications.