Spacecraft Materials and the Chemistry of Space Exploration

This artist rendering illustrates NASA's Cassini spacecraft flying through the plume on Saturn's moon Enceladus. Image credit: NASA/JPL-Caltech
Overview
In this lab activity, students will become materials scientists for a day. Working with NASA to design a satellite or a rover means understanding the properties of metals under conditions very unlike those on Earth. Which material should we use to construct a rover going to a planet like Venus? What if we were traveling to an icy planet or even underwater? The answer can be found in chemistry!
Management
Students should wear safety goggles and gloves throughout the lab. For the aluminum sample, it can be helpful to give the sample a quick polish with steel wool or a brush before student use. This is to remove any aluminum oxide that forms on the surface, as aluminum is fairly reactive (but still safe to handle).
Be ready to address student misconceptions during the observation phase. Once the copper is reacted out of the blue copper (II) solution, it will appear as brown elemental copper on the surface of the metal samples. This may lead students to think that their metal samples are rusting. This is an opportunity to discuss and clarify that rust is iron oxide, which is not present in our chemical reaction.
Background

This artist rendering illustrates a conceptual design for a potential future mission that would land a robotic probe on the surface of Jupiter's icy moon Europa. A Europa lander would need to withstand radiation, frigid temperatures, ice and potentially mist from possible plumes jetting from the moon's surface.
When we think about sending a spacecraft to another planet or into space, we ask questions like, “What will it look for?” or “How can we get it to its destination?” But when was the last time we asked, “What will it be made of?” This question is extremely important, as the material we choose needs to be suitable for the mission’s goals and locations. Imagine if we sent a satellite to a planet that can reach high temperatures, like Mercury, only to have it melt upon arrival! The study of how materials react to chemicals or conditions is called material science, and it is a very important field within chemistry for NASA scientists. For example, the New Horizons spacecraft, which was designed to fly by Pluto, was constructed of aluminum because it’s cheap and lightweight, which meant less fuel was needed to launch it into space. The Mars Curiosity rover, on the other hand, used a suspension system built largely out of titanium not just because it is strong, but also resistant to radiation. Similarly, titanium was used to protect the science instruments aboard the Juno spacecraft from the even more intense radiation at Jupiter.

It’s important to note that just because metals may look and feel strong it doesn’t mean they are. They can be highly reactive to other chemicals or environments. Maybe you’ve seen rust on a car or bicycle when iron is oxidized to form iron oxide. Imagine if you spent years designing a spacecraft only to have it be covered in rust when it arrived at its destination. Luckily, metals react very predictably, so if we know what chemicals are present at our destination, we can plan accordingly.
Procedures
Important safety note: Be
sure all students are wearing the appropriate personal protective equipment,
including goggles and gloves, before beginning.
1. Pour approximately 5-10mL of copper (II) sulfate solution into each test tube; one for every metal sample that will be explored.
2. Have students note the physical characteristics of each metal to be reacted (e.g., color, shape, hardness).
3. In each test tube, place a metal sample and begin recording observations. Be sure to note any changes at 0, 5 and 10 minutes that may indicate a chemical reaction (e.g., bubbles, temperature change, color change, etc.).
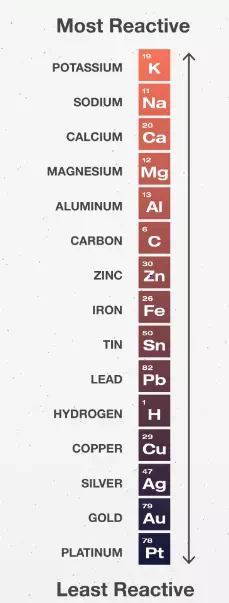
4. Rank the metals in order of reactivity.
5. If students are performing this as a lab activity, have them complete the student worksheet.
6. Clean up. Put all solid products in the waste bucket. Pour any liquid waste in the sink with running water and rinse the test tubes. As students are cleaning, have them observe the color and composition of the metals at the end of the reaction.
Discussion
· From what you observed, which materials would be best for a spacecraft going to a planet that contains copper (II) sulfate? What is your reasoning?
· From what you observed of the reactivity of metals, do you believe that the materials you used today generally exist in nature in their elemental form? Why or why not? Hint: Think about any changes you saw before you added the metals to the solution.
· In many applications here on Earth we use alloys – two or more elements combined to give us a mixture. Steel, for example, is a mixture of iron and carbon. Discuss why we might use alloys instead of the pure elements. How might an alloy of zinc combined with copper (called brass) be beneficial for use in our new rover design?
Assessment
- What was metal reactivity order, from highest to lowest?
- Explain why putting zinc into
magnesium sulfate would NOT produce a reaction.
Zn + MgSO4 ----> no reaction - Use the activity series of metals list
to predict whether or not the following reactions will occur.
Zn + PbSO4 ---->
Cu + FeSO4 ---->
Al + AgNO3 ---->
· The clouds on Venus contain droplets of sulfuric acid (H2SO4). If we were building a satellite or a rover for Venus, which metals should we use? Which ones should we avoid? Explain your reasoning.
· It may be difficult or expensive to build satellites and rovers entirely out of unreactive metals. Other factors may come into play, such as cost, weight or melting point. Discuss some pros and cons of some of the less reactive metals as candidates for your spacecraft. How could you incorporate more reactive metals into a design without having to worry about your rover dissolving before the mission is complete.
Extensions
As mentioned above, remind students that the process of rusting is different than the single replacement reaction they are observing. In the latter, one metal comes in, one metal goes out. In the former, the metal reacts (in this case with oxygen) to form a new compound where the two combine.
As a whole class demonstration or for students who finish early, rusting, or corrosion, can be demonstrated using steel wool. Using clean, dry test tubes, prepare the following:
1. A test tube containing 20mL of water
2. A test tube containing 20mL of water plus a tablespoon of table salt
3. A test tube containing 20mL of hydrogen peroxide
4. A test tube containing 20mL of hydrogen peroxide plus a tablespoon of table salt
To each test tube, add a piece of steel wool. You may need to submerge the sample with a glass rod (not a student’s pencil!). Make initial observations and have students revisit the samples the following day. The salt allows for the electrons to move throughout the solution and the hydrogen peroxide provides oxygen to result in the iron oxide rust. This demonstration and the activity series lab use oxidation-reduction of metals but are different types of reactions. Both need to be taken into consideration when constructing devices to explore worlds with varying chemical compositions.