The Chemistry and Physics of Fire
Fire is a manifestation of uncontrolled combustion. It involves combustible materials which are found around us in the buildings in which we live, work and play, as well as a wide range of gases, liquids and solids which are encountered in industry and commerce. They are commonly carbon-based, and may be referred to collectively as fuels in the context of this discussion. Despite the wide variety of these fuels in both their chemical and physical states, in fire they share features that are common to them all. Differences are encountered in the ease with which fire can be initiated (ignition), the rate with which fire can develop (flame spread), and the power that can be generated (rate of heat release), but as our understanding of the science of fire improves, we become better able to quantify and predict fire behaviour and apply our knowledge to fire safety in general. The purpose of this section is to review some of the underlying principles and provide guidance to an understanding of fire processes.
Basic Concepts
Combustible materials are all around us. Given the appropriate circumstances, they can be made to burn by subjecting them to an ignition source which is capable of initiating a self-sustaining reaction. In this process, the “fuel” reacts with oxygen from the air to release energy (heat), while being converted to products of combustion, some of which may be harmful. The mechanisms of ignition and burning need to be clearly understood. Most everyday fires involve solid materials (e.g., wood, wood products and synthetic polymers), although gaseous and liquid fuels are not uncommon. A brief review of the combustion of gases and liquids is desirable before some of the basic concepts are discussed.
Diffusion and premixed flames
A flammable gas (e.g., propane, C3H8) can be burned in two ways: a stream or jet of gas from a pipe (cf. the simple Bunsen burner with the air inlet closed) can be ignited and will burn as a diffusion flame in which burning occurs in those regions where gaseous fuel and air mix by diffusive processes. Such a flame has a characteristic yellow luminosity, indicating the presence of minute soot particles formed as a result of incomplete combustion. Some of these will burn in the flame, but others will emerge from the flame tip to form smoke. If the gas and air are intimately mixed before ignition, then premixed combustion will occur, provided that the gas/air mixture lies within a range of concentrations bounded by the lower and upper flammability limits (see table 41.1). Outside these limits, the mixture is non-flammable. (Note that a premixed flame is stabilized at the mouth of a Bunsen burner when the air inlet is open.) If a mixture is flammable, then it can be ignited by a small ignition source, such as an electrical spark. The stoichiometric mixture is the most readily ignited, in which the amount of oxygen present is in the correct proportion to burn all the fuel to carbon dioxide and water (see accompanying equation, below, in which nitrogen can be seen to be present in the same proportion as in air but does not take part in the reaction). Propane (C3H8) is the combustible material in this reaction:
C3H8 + 5O2 + 18.8N2 = 3CO2 + 4H2O + 18.8N2
Table 41.1 Lower and upper flammability limits in air
|
|
Lower flammability limit (% by volume) |
Upper flammability limit (% by volume) |
|
Carbon monoxide |
12.5 |
74 |
|
Methane |
5.0 |
15 |
|
Propane |
2.1 |
9.5 |
|
n-Hexane |
1.2 |
7.4 |
|
n-Decane |
0.75 |
5.6 |
|
Methanol |
6.7 |
36 |
|
Ethanol |
3.3 |
19 |
|
Acetone |
2.6 |
13 |
|
Benzene |
1.3 |
7.9 |
An electrical discharge as small as 0.3 mJ is sufficient to ignite a stoichiometric propane/air mixture in the reaction illustrated. This represents a barely perceptible static spark, as experienced by someone who has walked across a synthetic carpet and touched a grounded object. Even smaller amounts of energy are required for certain reactive gases such as hydrogen, ethylene and ethyne. In pure oxygen (as in the reaction above, but with no nitrogen present as a diluent), even lower energies are sufficient.
The diffusion flame associated with a flow of gaseous fuel exemplifies the mode of burning that is observed when a liquid or solid fuel is undergoing flaming combustion. However, in this case, the flame is fed by fuel vapours generated at the surface of the condensed phase. The rate of supply of these vapours is coupled to their rate of burning in the diffusion flame. Energy is transferred from the flame to the surface, thus providing the energy necessary to produce the vapours. This is a simple evaporative process for liquid fuels, but for solids, enough energy must be provided to cause chemical decomposition of the fuel, breaking large polymeric molecules into smaller fragments which can vaporize and escape from the surface. This thermal feedback is essential to maintain the flow of vapours, and hence support the diffusion flame (figure 41.1). Flames can be extinguished by interfering with this process in a number of ways (see below).
Figure 41.1 Schematic representation of a burning surface showing the heat and mass transfer processes

Heat transfer
An
understanding of heat (or energy) transfer is the key to an understanding of
fire behaviour and fire processes. The subject deserves careful study. There
are many excellent texts to which one may turn (Welty, Wilson and Wicks 1976;
DiNenno 1988), but for the present purposes it is necessary only to draw
attention to the three mechanisms: conduction, convection and radiation. The
basic equations for steady-state heat transfer (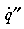 ) are:
) are:
Conduction: 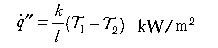
Convection: 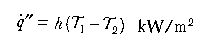
Radiation: 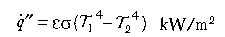
Conduction is relevant to heat transfer through solids; (k is a material property known as thermal conductivity (kW/mK ) and l is the distance (m) over which the temperature falls from T1 to T2 (in degrees Kelvin). Convection in this context refers to the transfer of heat from a fluid (in this case, air, flames or fire products) to a surface (solid or liquid); h is the convective heat transfer coefficient kW/m2K) and depends on the configuration of the surface and nature of the flow of fluid past that surface. Radiation is similar to visible light (but with a longer wavelength) and requires no intervening medium (it can traverse a vacuum); ε is the emissivity (efficiency by which a surface can radiate), σ is the Stefan-Boltzman constant (56.7 x 10-12 kW/m2K4). Thermal radiation travels at the speed of light (3 x 108 m/s) and an intervening solid object will cast a shadow.
Rate of burning and rate of heat release
Heat transfer from flames to the surface of condensed fuels (liquids and solids) involves a mixture of convection and radiation, although the latter dominates when the effective diameter of the fire exceeds 1 m. The rate of burning (m, (g/s)) can be expressed by the formula:
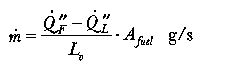
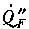 is the heat flux from the flame to the surface (kW/m2);
is the heat flux from the flame to the surface (kW/m2); is the heat loss from the surface (e.g., by radiation, and
by conduction through the solid) expressed as a flux (kW/m2); Afuel is
the surface area of the fuel (m2); and Lv is the
heat of gasification (equivalent to the latent heat of evaporation for a
liquid) (kJ/g). If a fire develops in a confined space, the hot smoky gases
rising from the fire (driven by buoyancy) are deflected beneath the ceiling,
heating the upper surfaces. The resulting smoke layer and the hot surfaces
radiate down to the lower part of the enclosure, in particular to the fuel
surface, thus increasing the rate of burning:
is the heat loss from the surface (e.g., by radiation, and
by conduction through the solid) expressed as a flux (kW/m2); Afuel is
the surface area of the fuel (m2); and Lv is the
heat of gasification (equivalent to the latent heat of evaporation for a
liquid) (kJ/g). If a fire develops in a confined space, the hot smoky gases
rising from the fire (driven by buoyancy) are deflected beneath the ceiling,
heating the upper surfaces. The resulting smoke layer and the hot surfaces
radiate down to the lower part of the enclosure, in particular to the fuel
surface, thus increasing the rate of burning:
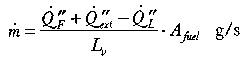
where 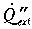 is the extra heat supplied by radiation from the upper part
of the enclosure (kW/m2). This additional feedback leads to greatly
enhanced rates of burning and to the phenomenon of flashover in enclosed spaces
where there is an adequate supply of air and sufficient fuel to sustain the
fire (Drysdale 1985).
is the extra heat supplied by radiation from the upper part
of the enclosure (kW/m2). This additional feedback leads to greatly
enhanced rates of burning and to the phenomenon of flashover in enclosed spaces
where there is an adequate supply of air and sufficient fuel to sustain the
fire (Drysdale 1985).
The rate of burning is moderated by the magnitude of the value of Lv, the heat of gasification. This tends to be low for liquids and relatively high for solids. Consequently, solids tend to burn much more slowly than liquids.
It has been argued that the most important single parameter which determines the fire behaviour of a material (or assembly of materials) is the rate of heat release (RHR) which is coupled to the rate of burning through the equation:
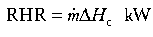
where ΔHc is the effective heat of combustion of the fuel (kJ/g). New techniques are now available for measuring the RHR at different heat fluxes (e.g., the Cone Calorimeter), and it is now possible to measure the RHR of large items, such as upholstered furniture and wall linings in large-scale calorimeters which use oxygen consumption measurements to determine the rate of heat release (Babrauskas and Grayson 1992).
It should be noted that as a fire grows in size, not only does the rate of heat release increase, but the rate of production of “fire products” also increases. These contain toxic and noxious species as well as particulate smoke, the yields of which will increase when a fire developing in a building enclosure becomes underventilated.
Ignition
Ignition of a liquid or solid involves raising the surface temperature until vapours are being evolved at a rate sufficient to support a flame after the vapours have been ignited. Liquid fuels can be classified according to their flashpoints, the lowest temperature at which there is a flammable vapour/air mixture at the surface (i.e., the vapour pressure corresponds to the lower flammability limit). These can be measured using a standard apparatus, and typical examples are given in table 41.2. A slightly higher temperature is required to produce a sufficient flow of vapours to support a diffusion flame. This is known as the firepoint. For combustible solids, the same concepts are valid, but higher temperatures are required as chemical decomposition is involved. The firepoint is typically in excess of 300 °C, depending on the fuel. In general, flame-retarded materials have significantly higher firepoints (see table 41.2).
Table 41.2 Flashpoints and firepoints of liquid and solid fuels
|
|
Closed cup flashpoint1 (°C) |
Firepoint2 (°C) |
|
Gasoline (100 Octane) (l) |
–38 |
– |
|
n-Decane (l) |
46 |
61.5 |
|
n-Dodecane (l) |
74 |
103 |
|
Polymethylmethacrylate (s) |
– |
»310 |
|
FR polymethylmethacrylate (s) |
– |
»377 |
|
Polypropylene (s) |
– |
»330 |
|
FR polypropylene (s) |
– |
»397 |
|
Polystyrene (s) |
– |
»367 |
|
FR polystyrene (s) |
– |
»445 |
l = liquid; s = solid.
1 By Pensky-Martens closed cup apparatus.
2 Liquids: by Cleveland open cup apparatus. Solids: Drysdale and Thomson (1994).
(Note that the results for the flame-retarded species refer to a heat flux of 37 kW/m2).
Ease of ignition of a solid material is therefore dependent on the ease with which its surface temperature can be raised to the firepoint, e.g., by exposure to radiant heat or to a flow of hot gases. This is less dependent on the chemistry of the decomposition process than on the thickness and physical properties of the solid, namely, its thermal conductivity (k), density (ρ) and heat capacity (c). Thin solids, such as wood shavings (and all thin sections), can be ignited very easily because they have a low thermal mass, that is, relatively little heat is required to raise the temperature to the firepoint. However, when heat is transferred to the surface of a thick solid, some will be conducted from the surface into the body of the solid, thus moderating the temperature rise of the surface. It can be shown theoretically that the rate of rise of the surface temperature is determined by the thermal inertia of the material, that is, the product kρc. This is borne out in practice, since thick materials with a high thermal inertia (e.g., oak, solid polyurethane) will take a long time to ignite under a given heat flux, whereas under identical conditions thick materials with a low thermal inertia (e.g., fibre insulating board, polyurethane foam) will ignite quickly (Drysdale 1985).
Ignition sources
Ignition is illustrated schematically in figure 41.2 (piloted ignition). For successful ignition, an ignition source must be capable not only of raising the surface temperature to the firepoint, or above, but it must also cause the vapours to ignite. An impinging flame will act in both capacities, but an imposed radiative flux from a remote source may lead to the evolution of vapours at a temperature above the firepoint, without the vapours igniting. However, if the evolved vapours are hot enough (which requires the surface temperature to be much higher than the firepoint), they may ignite spontaneously as they mix with air. This process is known as spontaneous ignition.
Figure 41.2 The scenario for piloted ignition
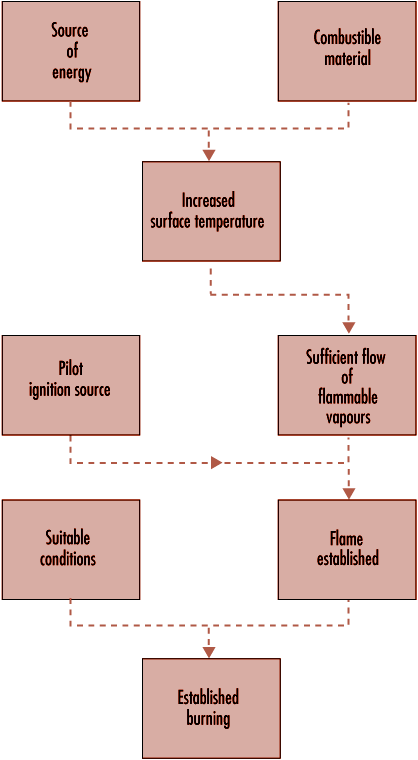
Table 41.3 Ignition sources
|
|
Examples |
|
Electrically powered equipment |
Electric heaters, hair dryers, electric blankets, etc. |
|
Open flame source |
Match, cigarette lighter, blow torch, etc. |
|
Gas-fuelled equipment |
Gas fire, space heater, cooker, etc. |
|
Other fuelled equipment |
Wood stove, etc. |
|
Lighted tobacco product |
Cigar, pipe, etc. |
|
Hot object |
Hot pipes, mechanical sparks, etc. |
|
Exposure to heating |
Adjacent fire, etc. |
|
Spontaneous heating |
Linseed oil-soaked rags, coal piles, etc. |
|
Chemical reaction |
Rare-e.g., potassium permanganate with glycerol |
It should be noted that smouldering cigarettes cannot initiate flaming combustion directly (even in common gaseous fuels), but can cause smouldering in materials which have the propensity to undergo this type of combustion. This is observed only with materials which char on heating. Smouldering involves the surface oxidation of the char, which generates enough heat locally to produce fresh char from adjacent unburnt fuel. It is a very slow process, but may eventually undergo a transition to flaming. Thereafter, the fire will develop very rapidly.
Materials which have the propensity to smoulder can also exhibit the phenomenon of self-heating (Bowes 1984). This arises when such a material is stored in large quantities and in such a way that heat generated by slow surface oxidation cannot escape, leading to a rise in temperature within the mass. If the conditions are right, this can lead to a runaway process ultimately developing into a smouldering reaction at depth within the material.
Flame spread
A major component in the growth of any fire is the rate at which flame will spread over adjacent combustible surfaces. Flame spread can be modelled as an advancing ignition front in which the leading edge of the flame acts as an ignition source for the fuel that is not yet burning. The rate of spread is determined partly by the same material properties that control the ease of ignition and partly by the interaction between the existing flame and the surface ahead of the front. Upward, vertical spread is the most rapid as buoyancy ensures that the flames flow upwards, exposing the surface above the burning area to direct heat transfer from the flames. This should be contrasted with spread over a horizontal surface when the flames from the burning area rise vertically, away from the surface. Indeed, it is common experience that vertical spread is the most hazardous (e.g., flame spread on curtains and drapes and on loose clothing such as dresses and nightgowns). The rate of spread is also affected by an imposed radiant heat flux. In the development of a fire in a room, the area of the fire will grow more rapidly under the increasing level of radiation that builds up as the fire progresses. This will contribute to the acceleration of fire growth that is characteristic of flashover.