Special extinguishing systems are used in cases where water sprinklers would not provide adequate protection or where the risk of damage from water would be unacceptable. In many cases where water damage is of concern, special extinguishing systems may be used in conjunction with water sprinkler systems, with the special extinguishing system designed to react at an early stage of fire development.
Water and water-additive special extinguishing systems
Water spray systems
Water spray systems increase the effectiveness of water by producing smaller water droplets, and thus a greater surface area of water is exposed to the fire, with a relative increase in heat absorption capability. This type of system is often chosen as a means of keeping large pressure vessels, such as butane spheres, cool when there is a risk of an exposure fire originating in an adjacent area. The system is similar to a sprinkler system; however, all heads are open, and a separate detection system or manual action is used to open control valves. This allows water to flow through the piping network to all spray devices that serve as outlets from the piping system.
Foam systems
In a foam system, a liquid concentrate is injected into the water supply before the control valve. Foam concentrate and air are mixed, either through the mechanical action of discharge or by aspirating air into the discharge device. The air entrained in the foam solution creates an expanded foam. As expanded foam is less dense than most hydrocarbons, the expanded foam forms a blanket on top of the flammable liquid. This foam blanket reduces fuel vapour propagation. Water, which represents as much as 97% of the foam solution, provides a cooling effect to further reduce vapour propagation and to cool hot objects that could serve as a source of re-ignition.
Gaseous extinguishing systems
Carbon dioxide systems
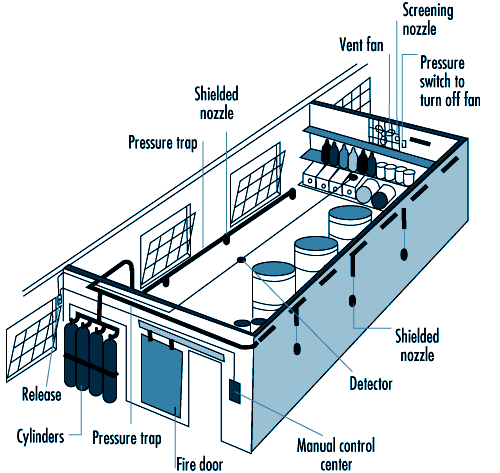
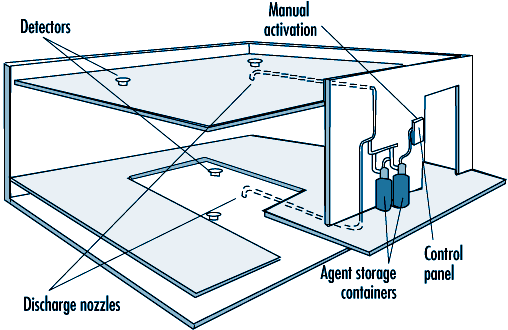
Carbon dioxide systems consist of a supply of carbon dioxide, stored as liquified compressed gas in pressure vessels (see figure 41.9 and figure 41.10). The carbon dioxide is held in the pressure vessel by means of an automatic valve that is opened upon fire by means of a separate detection system or by manual operation. Once released, the carbon dioxide is delivered to the fire by means of a piping and discharge nozzle arrangement. Carbon dioxide extinguishes fire by displacing the oxygen available to the fire. Carbon dioxide systems can be designed for use in open areas such as printing presses or enclosed volumes such as ship machinery spaces. Carbon dioxide, at fire-extinguishing concentrations, is toxic to people, and special measures must be employed to ensure that persons in the protected area are evacuated before discharge occurs. Pre-discharge alarms and other safety measures must be carefully incorporated into the design of the system to ensure adequate safety for people working in the protected area. Carbon dioxide is considered to be a clean extinguishant because it does not cause collateral damage and is electrically non-conductive.
Figure 41.9 Diagram of a high-pressure carbon dioxide system for total flooding
Figure 41.10 A total flooding system installed in a room with a raised floor
Inert gas systems
Inert gas systems generally use a mixture of nitrogen and argon as an extinguishing medium. In some cases, a small percentage of carbon dioxide is also provided in the gas mixture. The inert gas mixtures extinguish fires by reducing oxygen concentration within a protected volume. They are suitable for use in enclosed spaces only. The unique feature offered by inert gas mixtures is that they reduce the oxygen to a low enough concentration to extinguish many types of fires; however, oxygen levels are not sufficiently lowered to pose an immediate threat to occupants of the protected space. The inert gases are compressed and stored in pressure vessels. System operation is similar to a carbon dioxide system. As the inert gases cannot be liquified by compression, the number of storage vessels required for protection of a given enclosed protected volume is greater than that for carbon dioxide.
Halon systems
Halons 1301, 1211 and 2402 have been identified as ozone-depleting substances. Production of these extinguishing agents ceased in 1994, as required by the Montreal Protocol, an international agreement to protect the earth’s ozone layer. Halon 1301 was most often used in fixed fire protection systems. Halon 1301 was stored as liquified, compressed gas in pressure vessels in a similar arrangement to that used for carbon dioxide. The advantage offered by halon 1301 was that storage pressures were lower and that very low concentrations provided effective extinguishing capability. Halon 1301 systems were used successfully for totally enclosed hazards where the extinguishing concentration achieved could be maintained for a sufficient time for extinguishment to occur. For most risks, concentrations used did not pose an immediate threat to occupants. Halon 1301 is still used for several important applications where acceptable alternatives have yet to be developed. Examples include use on-board commercial and military aircraft and for some special cases where inerting concentrations are required to prevent explosions in areas where occupants could be present. The halon in existing halon systems that are no longer required should be made available for use by others with critical applications. This will militate against the need to produce more of these environmentally sensitive extinguishers and help protect the ozone layer.
Halocarbon systems
Halocarbon agents were developed as the result of the environmental concerns associated with halons. These agents differ widely in toxicity, environmental impact, storage weight and volume requirements, cost and availability of approved system hardware. They all can be stored as liquified compressed gases in pressure vessels. System configuration is similar to a carbon dioxide system.
Design, Installation and Maintenance of Active Fire Protection Systems
Only those skilled in this work are competent to design, install and maintain this equipment. It may be necessary for many of those charged with purchasing, installing, inspecting, testing, approving and maintaining this equipment to consult with an experienced and competent fire protection specialist to discharge their duties effectively.