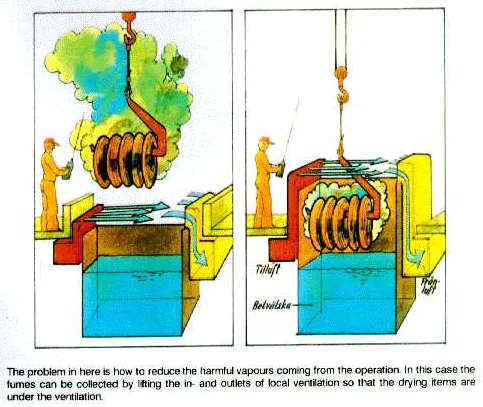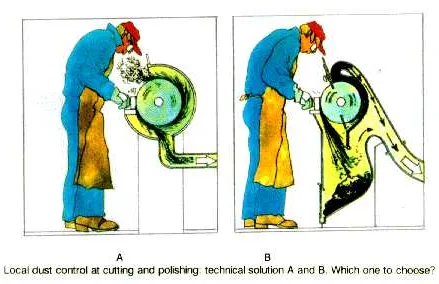
Dust may be just a nuisance, and the danger depends on the type of material in the dust, and on the amount and the size of the particles.
The smaller the particle is the deeper it will penetrate into the lungs with the inhaled air, thereby passing the defensive systems of the lungs. This type of dust is invisible to the eye and identified using microscope technique. Such dust can accumulate in the lungs over a long period of time and cause a lung disease called pneumoconiosis, which is a common incapacitating occupational disease. Dusts containing crystalline silica or asbestos are particularly dangerous.
Sand and many types of stone contain crystalline silica, as do many ores, concrete, ceramics and diatomite. Processing of these materials creates dust with result of silica accumulating in the lungs. This may lead after years to a incurable lung disease, even though the exposure has been stopped years before.
Asbestos is a natural mineral fibre which is very resistant to fire and to many chemicals. Asbestos fibres are very strong and thin. Asbestos exists in various forms and names: chrysotile, crocidolite, amosite, anthophyllite, actinolite and tremolite asbestos. Chrysotile is used in isolating materials, protective carpets and clothes.
The dust penetrates the lungs destroying the lung tissue. This condition is called asbestosis. Asbestos can also cause lung cancer. The risk of cancer is many times higher if the asbestos exposure is combined with smoking. Many countries have restricted or banned the use of asbestos.
Exposure to metal fumes can cause damage to the body. `Metal fume fever' is a known health effect when metal fumes, often containing zinc, are inhaled. It usually appears on the day following that of the exposure.
Gases do not necessarily have a warning odour at a dangerous concentration. The odour may be apparent only at very high concentration in the air. Gases may have an irritating effect, or they may enter the blood circulation and cause internal damage.
Sulphur oxides, nitrogen oxides, chlorine and ammonia are toxic gases that are corrosive and irritating to the respiratory system. They are widely used in industry. Phosgene is formed when solvents containing chlorine, such as "TRI" (1,1,2- trichloroethylene), come into contact with hot surfaces or flames. Phosgene can be deadly poisonous even before the odour is detected.
Carbon monoxide is a toxic, odourless, colourless gas which is formed by the incomplete burning of materials of organic origin. It may enter the blood circulation. Some gases can pass through the skin, for example, hydrogen cyanide.
Most solvents are liquid organic chemicals. They are used because of their ability to dissolve other substances, particularly fat and grease, which are insoluble in water. Many of them evaporate rapidly at ambient temperatures. They are often flammable and may ignite by heat from smoking, welding or static electricity. Vapours move with air currents and can ignite even by a distant heat source.
Inhalation is the most common way for solvents to enter the body, but some of them penetrate intact healthy skin. Once in the blood stream a solvent can be transported to different organs, such as the brain and liver.
Solvents have different effects on humans, depending on their evaporation rate and their solubility in water. The risks of health effects depend on the period of exposure and the concentration of the solvent in the inhaled air.
Many solvents have a narcotic effect; they may cause dizziness, headache, reduced comprehension or tiredness. They may also irritate the eyes and the respiratory tract. Frequent skin contact defats the protective layer of the skin causing irritation. Some solvents are very hazardous to the liver, kidneys, bone marrow or nervous system. Benzene, carbon tetrachloride and carbon disulphide belong to the category of solvents which should be substituted with less dangerous ones.
Metals can enter the body in the form of dust and fumes (in grinding or welding) or even through the skin. One of these is tetraethyl lead, which is used as an anti-knocking agent in petrol. Mercury vapours are often inhaled, as this liquid metal evaporates readily at room temperatures.
Lead is used in various industries: battery, glass and mining industries, cable manufacturing, foundries and in printing works. Steel constructions are protected with anti-corrosive paint containing lead, which may be released during welding operations, for example, on ships.
Mercury is present in many pesticides and pickling baths. In the environment, it may accumulate in fish. Mercury poisoning has serious effects on the nervous system.
Nickel is present with other metals in various alloys. Nickel and its compounds are known to be sensitizers. Once a person has had an allergic reaction to nickel, the reaction reoccurs following the contact with very small amounts of nickel used in products such as leather, cement, or door handles. Some compounds of nickel can cause cancer.
Chromium compounds, particularly chromates and bichromates, are widely used in industry. Cement contains small amounts of chromium compounds. These compounds can cause allergy and even lung cancer. Unlike cobalt and nickel, pure metallic chromium does not cause allergy. Chromium compounds may cause birth defects if mothers are exposed to these compounds during pregnancy.
Arsenic compounds are used in pesticides, insecticides and in some colouring materials. Chronic arsenic poisoning can start with irritation to the respiratory system, inflammation of the eyes, or skin problems, followed by damage in nervous system. Arsenic and its compounds can cause cancer.
Strong acids and bases are mostly used as water solutions. They are corrosive to human tissue. Working with acids or bases can give rise to mists which have the same corrosive properties as the solutions.
When acids and bases are mixed with each other the phenomena of neutralization occurs, usually with strong production of heat. The heat production has particularly serious effects when water is added to concentrated sulphuric acid: the heat will splash the highly corrosive liquid up, risking injury to the worker.
Some acids are explosive when in contact with organic material, such as sawdust.
Serious damage can result when treating metal pieces in a acid bath. The bath may contain more than one acid in a mixture and may release flammable hydrogen gas, as well as acid mist, when a piece of metal is placed in it.
Phosphoric acid is used to treat metals. When in contact with hot surfaces, phosphoric acid can give off poisonous gases. Ammonia, sodium and potassium hydroxides are commonly used bases. They are corrosive to human tissue in such a way that a certain period of time is required before the corrosive feeling is sensed. Bases penetrate the skin and cause deep sores. They are difficult to wash away. Dilute water solutions are irritating.
Sodium and potassium hydroxides are used, for example, in hot degreasing baths for cleaning metals.
Pesticides are intended to destroy or control pests of all kind. They are used in industry, for example, to impregnate wood, and in agriculture to control insects, weed, fungi, and rats. These are many different types of pesticide compounds and they are used also as mixtures.
Some countries apply restrictions in using certain compounds, and the use of some of them is completely banned because of their serious adverse effects. In Europe, the list of banned pesticides includes compounds such as inorganic mercury compounds, camphechlor, chlordane, dieldrin, DDT, HCH (lindane), heptachlor, hexachlorobenzene, and nitrofen.
Insecticides are divided into following broad groups:
Organophosphorous compounds
These are often acutely poisonous to insects and to humans. They can damage the nervous system and even cause death. They are effective even at low concentrations. Dichlorvos, demeton, parathion and thioazin belong to this group.
Organochlorine compounds
These compounds have a lower acute poisoning effect than organophosphorous compounds. They decompose slowly and can therefore accumulate in the environment and in the body. Aldrin, dieldrin, heptachlor, and DDT belong to this group.
Carbamates are insecticides and fungicides
They are poisonous to humans causing same type of symptoms as organophosphates. Dithiocarb and carbaryl belong to this group.