3D-Printed Models Applied in Medical Research Studies
In recent years, the use of three-dimensional (3D) files generated in noninvasive-imaging technologies (NITs) combined with additive manufacturing (AM) technologies has increased exponentially. NITs are adopted to assist diagnosis in medicine allowing the physician to visualize and define relevant internal structures of the human body, as well as the conversion of the visualized information into a set of digital medical 3D data.
Eight selected experiments developed by a multidisciplinary team of physicians, designers, and physicists are described in order to highlight the potential of the combination of AM and NITs, demonstrating that 3D representation enhances the understanding for the diagnosis and treatment in medicine.
Four NITs were adopted so as to generate 3D imaging files: 3D ultrasound (3DUS), magnetic resonance imaging (MRI), computerized tomography (CT), and micro-computed tomography (micro-CT). The routine of imaging acquisition and the AM systems are similar regarding their logical method based on the virtual “slicing” of an object, generating an amount of layers according to the thickness recommended [1].
The consecutive construction of the projected 3D computer-aided design (CAD) model can be reached throughout the superimposition of those same layers. The additive materialization process begins with the 3D CAD model, which is then “sliced” into layers that contain spatial references to guide later the deposit of selected materials, layer by layer, resulting on a physical 3D model.
The actual additive method is based upon the successive overlapping of thin layers of specific material substances, according to the appropriate technical method, and is carried out by transforming the 3D files into an STL (Standard Triangulation Language) extension, which consists basically of X, Y, and Zcoordinates. Once the STL file is generated, the next step is the horizontal slicing of the whole 3D volumetric file using software appropriate to the specific hardware being used, and calculating the supporting structures when necessary. The building process starts with the sequential deposition of layers of material, the layer width ranging from microns to fractions of millimeters, depending on the technology chosen [1].
This process is then followed by a post-processing stage, an essential procedure in all current AM technologies, where the model has to be cleaned to remove the support material and/or residues used during the building process. In the case of some AM processes that work by using a laser beam to harden photosensitive materials, it is also necessary to position and expose the model inside an ultraviolet light camera in order to solidify the model completely.
There are presently diverse systems of AM/3D printing technologies commercially available. Although they use different material processes, they are all based on the principle of physical materialization by layer deposition. One of the most important characteristics of the additive manufacturing technology is its capability of construction parts with any geometrical intricacy, a process in which subtractive technologies as CNC equipment are sometimes limited and time consuming [1–3].
3D models in cardiology research projects
The use of imaging techniques in cardiology had its beginning with the advent of chest X-ray, in which a topographic image of the heart could be done in different projections (1). In this type of image, volumetric structures appeared overlapping each other in the X-ray film. Multiple projections helped the analysis of cardiac structures and the location of abnormalities. Another radioactivity utility was the use of radiotracers and detection chambers—developing the planar Gamma Cameras, in which two-dimensional images were generated by detecting gamma radiation emitted by the radiotracer distributed through the heart [4].
The idea of generating images as sequences slices of the heart gave rise to tomographic techniques. In this field, computing tomography was developed with the use of radiation generated by an electrical current: spect scintigraphy imaging, using the radiotracers, and magnetic resonance imaging, by acquiring energy emitted by accommodation of the nuclear spins. The possibility of three-dimensional reconstruction using two-dimensional tomographic images provided remarkable progress in the study of heart anatomy and diseases [5].
By the same principle of using two-dimensional images for three-dimensional reconstruction, the 3D echocardiography was developed. With this technique, it is possible to present the heart images in three dimensions. In addition, the heart movement is displayed, adding important information about the heart function, once it is a dynamic organ with constant movement [6–8].
In assessing the evolution of image acquisition techniques, topographic, two-dimensional slices, three-dimensional reconstruction, 3D moving images, we can see that fewer mental process and imagination is required to design an imaginary volumetric structure through their 2D projections. This fact seems to be important, because each individual has different abilities to mental reconstruction of a 3D structure. Such variability can bring a discrepancy in the evaluation of the same case by different physicians. For example, during surgical planning, there is no guarantee that the tomographic images analysis, by the surgeon and the radiologist, generated similar mental conceptions in the mind of each of these professionals [1, 9, 10].
As a next step, the construction of patient-specific 3D heart models, using rapid prototyping techniques, probably, will bring benefits to the cardiovascular science. Recent publications have pointed to the possibilities generated by this technology in cardiology. In education and medical training, 3D printing of heart structures can assist in improving the learning curve. On the diagnostic process, probably, the congenital and valvular heart disease may be better studied with the printing of physical models. In the treatment, especially in surgical interventions, patient-specific three-dimensional models can help as a pre-step procedure for a more assertive surgical planning. Perhaps, it can be said that the three-dimensional physical models, in a certain way, standardize the imaginary mental process of 3D reconstruction. Furthermore, in these models, the structures are displayed in a three-dimensional space, unlike the conventional 3D images displayed on a two-dimensional screen. The 3D environment is also present in holographic projections and virtual reality simulations, where the structures are manipulated and analyzed in a 3D virtual space. Although promising, the calibration and quality control of the equipment that are involved in the rapid prototyping technique are key factors. In each process step, image acquisition, digital file segmentation, 3D printing, and systematic errors can be added, resulting in a physical model that do not represent the real organ [10, 11].
Figure 1 shows a physical model, of a healthy heart of a medium height man, 50 years old. The digital archive of heart images was generated by performing an angio-CT scan, Somatom Sensation 64 × 0.6 mm (Siemens Inc., Germany).
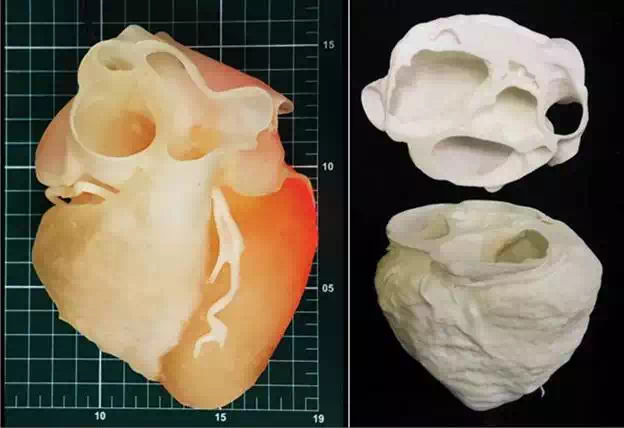
FIGURE 1.
A. 3D model of human heart from CT scanner with “arduino” electronic board controlling led inside the model to allow the visualization of different densities of the heart positioned on measuring scale table (scale reference: 1 cm2)—(3D printed in photosensible resin—PROJET 3510 HD Plus—3D Systems)—Núcleo de Experimentação Tridimensional—NEXT PUC-Rio; B: Same 3D file printed in two parts to allow the visualization of the internal structures (3D printed in polyamide at EOS P110)—Instituto Nacional de Tecnologia—Laboratório de Modelos Tridimensionais—Ministério da Ciência, Tecnologia e Inovação.
Aortic valve can be affected by many diseases that culminate with the necessity of a heart surgery, for aortic valve replacement. In this surgical procedure, the native aortic valve, dysfunctional, is excised and a biological or metallic prosthesis is implanted in the aortic annulus. After this surgery, several complications may occur. When the suture fixing the prosthetic valve in the aortic annulus breaks, a hole is formed between the prosthetic ring and the aortic annulus, a process named as paraprosthetic leak. With this complication, during ventricular diastole, blood present in the aortic root returns to the left ventricle, causing volume overload that can result with a severe left ventricular dysfunction.
Figure 2A presents a physical model, in full scale, of a heart segment in which there is a paraprosthetic leak around a biological aortic valve prosthesis. This hole measures approximately 1 cm. This model was carried out with the purpose of planning the percutaneous procedure that would be done to fix this abnormality. In Figure 2B and C, the implantation process of an occluder may be noted within the paraprosthetic leak. In this case, the physical model can help the physician to detect the spatial location of the defect to be corrected before the procedure is started. Besides that, the physical model can be used to test many types and sizes of percutaneous occluders. The digital archive of heart images was generated by performing an angio-CT scan, Somatom Sensation 64 × 0.6 mm (Siemens Inc., Germany).
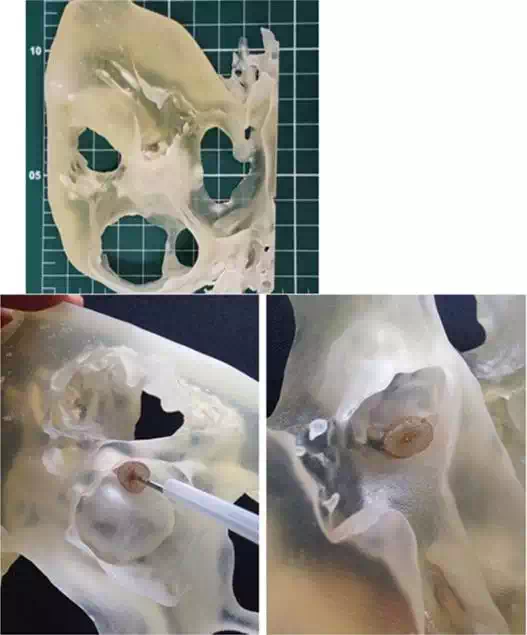
FIGURE 2.
A. 3D model of heart section from CT scanner files positioned on measuring scale table (scale reference: 1 cm2); B and C: detail highlighting paravalvular leak beside aortic bioprosthesis (3D printed in photosensible resin—PROJET 3510 HD Plus - 3D Systems)—Núcleo de Experimentação Tridimensional—NEXT PUC-Rio.
Aortic aneurysms
In the field of vascular surgery, particularly in the case of aortic stent grafting of the treatment of aortic aneurysms, there is already a commercially available product in which the stent graft is custom-made for each patient with unusual complex anatomy relaying on the 3D printing technology. To provide the surgeon with training, he receives in advance a prototype of the custom-made stent graft and a 3D model of the patients aorta. If needed, modifications can be suggested until there is agreement between the engineering and the medical team [12, 13].
The evolving technology allows for flexible and malleable models that are fairly realistic in its shape (Figure 3A and B). Another practical application already in use is medical training itself, in which the training vascular surgeon can learn the so-called endovascular techniques by deploying stents and stent grafts in 3D artery models (Figure 3C and D). These techniques are normally learned during hundreds of hours of “hands-on” training under staff supervision, which carry along, for both patients and surgeons, extra doses of radiation, required to perform these interventional radiology procedures. The current underdeveloping 3D training models do not require radiation and give the training surgeon a 3D hands-on feeling and vision of the devices itself, since we can print transparent flexible models, while the actual endovascular procedures provide only with the regular 2D images of the monitor screen (Figure 4).
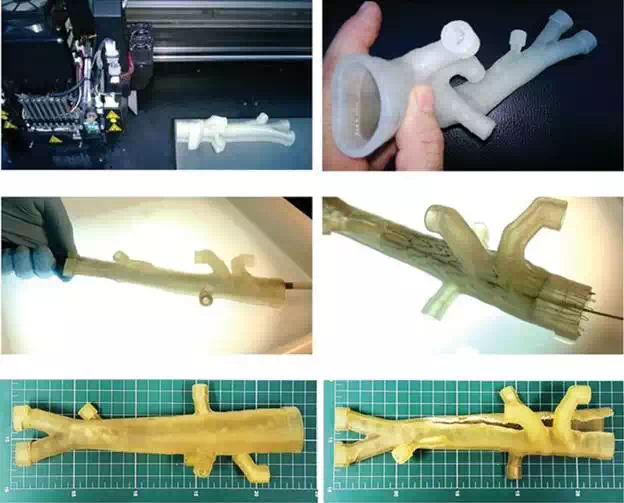
FIGURE 3.
A and B. Printing process and the flexible 3D aorta model; C and D: deployment of an aortic stent graft into an aortic model, serving as educational resource for training vascular surgeons; E: model positioned on measuring scale table (scale reference: 1 cm2); F: model damaged after tests. (3D printed in flexible clear resin on OBJET Connex 350)—Instituto Nacional de Tecnologia—Laboratório de Modelos Tridimensionais—Ministério da Ciência, Tecnologia e Inovação.

FIGURE 4.
A–D. Sequence of the development of a 3D flexible model of aorta from CT scanner files; E: 3D printed flexible model of aorta positioned on measuring scale table (scale reference: 1 cm2), damaged after tests (3D Printed in “Tango black” flexible resin on OBJET Connex 350)—Instituto Nacional de Tecnologia—Laboratório de Modelos Tridimensionais—Ministério da Ciência, Tecnologia e Inovação.; F: variant colored rigid 3D printed model of aorta (3D printed in thermoplastic ABS—Stratasys U-Print-Fused Deposition Modeling)—Núcleo de Experimentação Tridimensional—NEXT PUC-Rio.
Mitral regurgitation
The mitral valve prolapse, the well-known entity, in some specific cases may be associated with mitral regurgitation. When mitral regurgitation reaches a high level, a large volume of blood returns to the left atrium during ventricular systole. In these cases, there may be important symptoms culminating with left ventricular dysfunction. When this occurs, a mitral regurgitation correction procedure is indicated. One surgical technique, already established for this condition, is the Quadrangular Resection. When a severe mitral regurgitation is caused by prolapse of the posterior leaflet, the Quadrangular Resection can be performed. In this surgical technique, part of valve leaflet tissue is excised, besides the confection of a suture in the mitral annulus. The objective of this procedure is to enable the full coaptation of the leaflets during ventricular systole, preventing blood regurgitation into the left atrium [7].
Figure 5A presents, in three projections, the mitral valve leaflets of a patient with prolapse of the posterior leaflet, which cause no coaptation and a regurgitant orifice. Again, as a step to plan the surgical procedure, a virtual simulation of Quadrangular Resection was performed in the digital file using Rhinoceros 5.0 (McNeel North America, USA). In this simulation, the amount of tissue that would be needed to be excised from the posterior mitral leaflet can be estimated (Figure 5B).
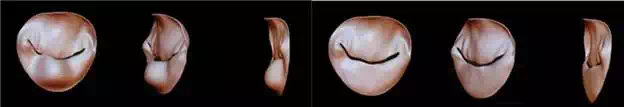
FIGURE 5.
3D virtual models of Quadrangular Resection in mitral valve repair—Núcleo de Experimentação Tridimensional—NEXT PUC-Rio.

FIGURE 6.
A–D. 3D printed model of mitral valve prolapse from 3D ultrasound (3D US)—printed in poliamide—(EOS P110)—Instituto Nacional de Tecnologia—Laboratório de Modelos Tridimensionais—Ministério da Ciência, Tecnologia e Inovação.
This file is also used for making the physical model, which can be seen from different angles in Figure 6A–D. The digital file of the mitral valve leaflets was generated by performing a 3D Echocardiography with Vivid E9 (GE Healthcare, USA).
Fetal medicine
Advances in image-scanning technology have led to vast improvements in fetal medicine [14–16].
In general, three main technologies are used to obtain images within the uterus during pregnancy—ultrasound, MRI, and CT. US is currently the primary method for fetal assessment during pregnancy because it is patient-friendly, useful, cost-effective, and considered to be safe. When ultrasound yields equivocal results, MRI can be used to help in diagnosis. CT can be used after 30 weeks of gestation only in cases of skeletal dysplasias [3, 17].
The development of ultrasound scanning during the 1960s opened a new window into the study of the fetus. Its introduction in the clinical obstetrics practice allowed the opportunity to make screening of many malformations (Figures 7–10).

FIGURE 7.
A and B. 3D printed hollow model of a head of a 32-week fetus from magnetic resonance imaging (MRI) highlighting fetal upper respiratory tract model positioned on measuring scale table (scale reference: 1 cm2)—3D printed in polyamide (EOS P110)––Instituto Nacional de Tecnologia—Laboratório de Modelos Tridimensionais—Ministério da Ciência, Tecnologia e Inovação.
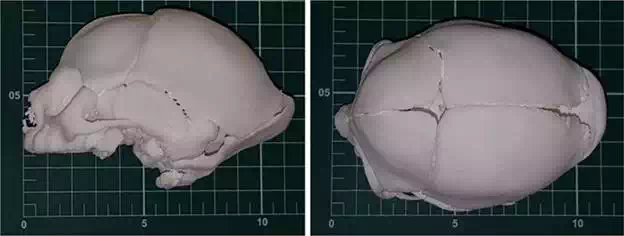
FIGURE 8.
A and B. 3D full-scale model of the skull of neonate with microcephaly from CT scanner, model positioned on measuring scale table (scale reference: 1 cm2)—3D printed in polyamide (EOS P110)––Instituto Nacional de Tecnologia—Laboratório de Modelos Tridimensionais—Ministério da Ciência, Tecnologia e Inovação.

FIGURE 9.
A and B. Fetal heart at 38 weeks, after autopsy, being positioned inside a micro-CT scanner (Zeiss Xradia Versa 510) and 3D virtual model generated from the DICOM micro-CT files—Department of Chemical and Materials Engineering—Rio de Janeiro, Brazil—DEQM/PUC-Rio and 3D printed models (3D Systems PROJET 3510 HD Plus) —Núcleo de Experimentação Tridimensional—NEXT PUC-Rio.
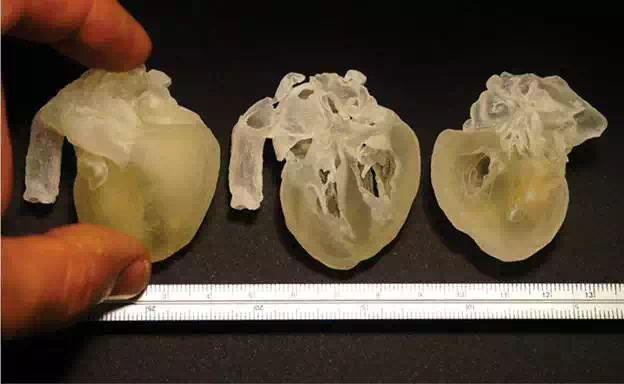
FIGURE 10.
3D printed full-scale model of human fetal heart (38 weeks) from micro-CT scanner files —SLA system (3D Systems PRO-7 JET 3510 HD Plus)—Núcleo de Experimentação Tridimensional—NEXT PUC-Rio.
MRI is a noninvasive procedure that has been utilized in obstetrics since the 1980s. It offers digital files with exceptional image contrast allowing a great visual comprehension of the fetus internal tissues. When the use of ultrasound detects or produces unexpected results, MRI is often adopted, since it provides further information about fetal malformations and conditions for which ultrasound cannot generate superior images [18].
MRI files can generate detailed characteristics of the soft tissues of the fetus body as the face, hands, or feet as well as internal body structures as aerial paths. The construction process of the 3D accurate virtual model starts with the 3D modeling volume built through the MRI slices sequentially mounted, followed by the segmentation process where the physician selects the important body parts to be analyzed that will then be reconstructed in 3D [19].
Fetal 3D physical models from 3DUS and MRI scan data have been used in blind pregnant women to improve maternal fetal attachment [20, 21].
Conclusion
3D printing has become a very useful and potentially transformative tool in a number of different fields, including medicine. It is an emerging technique with a variety of medical applications such as surgical planning and training, implant design, biomedical research, and medical education. There is an enormous potential of the technique and in the near future growing utilization and development of new applications in the fields of individual patient care, as well as academic and research activities will occur.
Researchers continue to improve existing medical applications that use 3D printing technology and to explore new ones. 3D printing provides a new didactic approach to learning when applied to medicine. The opportunity to prospect this paradigm shift in knowledge that occurs in the transition from 2D to 3D enables doctors to learn more inductively through the 3D modeling as a learning tool and improve patient’s care.