Nuclear
Reactor Coolant
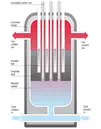
Nuclear reactor coolant is
coolant in a nuclear reactor that is used to remove heat from the nuclear
reactor core and transfer it to electrical generators and the environment.
Frequently a chain of two coolant loops is used because the primary coolant
loop takes on short-term radioactivity from the reactor.
Almost
all currently operating nuclear power plants are light water reactors using
ordinary water under high pressure as coolant and neutron moderator. About 1/3
are boiling water reactors where the primary coolant undergoes phase change to
steam inside the reactor. About 2/3 are pressurized water reactors at even
higher pressure. Current reactors stay under the critical point at around 374
°C and 218 bar where the distinction between liquid and gas disappears, which
limits thermal efficiency, but the proposed supercritical water reactor would
operate above this point.
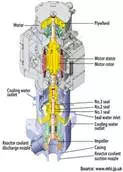
Fast
reactors have a high power density and do not need neutron moderation. Most
have been liquid metal cooled reactors using molten sodium. Lead and other metals
have also been proposed and occasionally used.
Molten
salts share with metals the advantage of low vapor pressure even at high
temperatures, and are less chemically reactive than sodium. Salts containing
light elements like FLiBe can also provide moderation. In the Molten-Salt
Reactor Experiment it even served as a solvent carrying the nuclear fuel.
Gases
have also been used as coolant. Helium is extremely inert both chemically and
with respect to nuclear reactions but has a low heat capacity, necessitating
rapid circulation. Carbon dioxide has also been used. Gases of course need to
be under pressure for sufficient density at high temperature.
|
COOLANT |
MELTING POINT |
BOILING POINT |
|
Light
water at 155 bar |
|
345
°C |
|
Mercury |
-38.83
°C |
356.73
°C |
|
NaK
eutectic |
-11
°C |
785
°C |
|
Sodium |
97.72
°C |
883
°C |
|
FLiBe |
459
°C |
1430
°C |
|
Lead |
327.46
°C |
1749
°C |
|
Lead-bismuth
eutectic |
123.5
°C |
1670
°C |