Coal Gasification
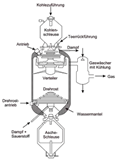
Coal gasification is the process of producing coal
gas, a type of syngas–a mixture of carbon monoxide (CO) and hydrogen (H2)
gas–from coal. Carbon monoxide, which is a combustible gas, was traditionally
used as a source of energy for municipal lighting and heat before the advent of
industrial-scale production of natural gas, while the hydrogen obtained from
gasification can be used for various purposes such as making ammonia, powering
a hydrogen economy, or upgrading fossil fuels. Alternatively, the coal gas
(also known as "town gas") can be converted into transportation fuels
such as gasoline and diesel through additional treatment via the Fischer-Tropsch process. This latter purpose of coal
gasification has been implemented by nations that have abundant sources of coal
but little to no petroleum reserves, as well as by nations seeking to decrease
their dependence on foreign sources of petroleum.
During gasification, the coal is blown through with oxygen
and steam (water vapor) while also being heated
(and in some cases pressurized). If the coal is heated by external heat sources
the process is called "allothermal", while
"autothermal" process assumes heating of
the coal via exothermal chemical reactions occurring inside the gasifier
itself. It is essential that the oxidizer supplied is insufficient for complete
oxidizing (combustion) of the fuel. During the reactions mentioned, oxygen and
water molecules oxidize the coal and produce a gaseous mixture of carbon
dioxide (CO2), carbon monoxide (CO), water vapour (H2O),
and molecular hydrogen (H2). (Some by-products like tar, phenols,
etc. are also possible end products, depending on the specific gasification
technology utilized.) This process has been conducted in-situ within natural
coal seams (referred to as underground coal gasification) and in coal
refineries. The desired end product is usually syngas (i.e., a combination of H2 +
CO), but the produced coal gas may also be further refined to produce
additional quantities of H2:
3C (i.e., coal) + O2 +
H2O → H2 +
3CO
If the refiner wants to produce alkanes (i.e., hydrocarbons
present in natural gas, gasoline, and diesel fuel), the coal gas is collected
at this state and routed to a Fischer-Tropsch reactor.
If, however, hydrogen is the desired end-product, the coal gas (primarily the
CO product) undergoes the water gas shift reaction where more hydrogen is
produced by additional reaction with water vapor:
CO + H2O → CO2 + H2
Although other technologies for coal gasification currently
exist, all employ, in general, the same chemical processes. For low-grade coals
(i.e., "brown coals") which contain significant amounts of water,
there are technologies in which no steam is required during the reaction, with
coal (carbon) and oxygen being the only reactants. As well, some coal
gasification technologies do not require high pressures. Some utilize
pulverized coal as fuel while others work with relatively large fractions of
coal. Gasification technologies also vary in the way the blowing is supplied.
"Direct blowing" assumes the coal and the oxidizer being supplied
towards each other from the opposite sides of the reactor channel. In this case
the oxidizer passes through coke and (more likely) ashes to the reaction zone
where it interacts with coal. The hot gas produced then passes fresh fuel and
heats it while absorbing some products of thermal destruction of the fuel, such
as tars and phenols. Thus, the gas requires significant refining before being
used in the Fischer-Tropsch reaction. Products
of the refinement are highly toxic and require special facilities for their
utilization. As a result, the plant utilizing the described technologies has to
be very large to be economically efficient. One of such plants called SASOL is
situated in the Republic of South Africa (RSA). It was built due to embargo
applied to the country preventing it from importing oil and natural gas. RSA is
rich in its own brown coal and was able to arrange the use of the well known high pressure "Lurgi"
gasification process developed in Germany in the first half of 20-th century.
"Reversed blowing" (as compared to the previous type
described which was invented first) assumes the coal and the oxidizer being
supplied from the same side of the reactor. In this case there is no chemical
interaction between coal and oxidizer before the reaction zone. The gas
produced in the reaction zone passes solid products of gasification (coke and
ashes), and CO2and H2O contained in the gas are
additionally chemically restored to CO and H2. As compared to the
"direct blowing" technology, no toxic by-products are present in the
gas: those are disabled in the reaction zone. This type of gasification has
been developed in the first half of 20-th century, along with the "direct
blowing", but the rate of gas production in it is significantly lower than
that in "direct blowing" and there were no further efforts of
developing the "reversed blowing" processes until 1980-s when a
Soviet research facility KATEKNIIUgol' (R&D
Institute for developingKansk-Achinsk coal
field) began R&D activities to produce the technology now known as
"TERMOKOKS-S" process. The reason for reviving the interest to this
type of gasification process is that it is ecologically clean and able to
produce two types of useful products (simultaneously or separately): gas
(either combustible or syngas) and middle-temperature coke. The former may be
used as a fuel for gas boilers and diesel-generators or as syngas for producing
gasoline, etc., the latter - as a technological fuel in metallurgy, as a
chemical absorbent or as raw material for household fuel briquettes. Combustion
of the product gas in gas boilers is ecologically cleaner than combustion of
initial coal. Thus, a plant utilizing gasification technology with the
"reversed blowing" is able to produce two valuable products of which
one has relatively zero production cost since the latter is covered by
competitive market price of the other. As the Soviet Union and itsKATEKNIIUgol' ceased to exist, the technology was adopted
by the individual scientists who originally developed it and is now being
further researched in Russia and commercially distributed worldwide. Industrial
plants utilizing it are now known to function in Ulaan-Baatar (Mongolia)
and Krasnoyarsk (Russia).