Biomass Gasification
Gasification is a process
that converts carbonaceous materials, such as coal, petroleum, biofuel, or
biomass, into carbon monoxide and hydrogen by reacting the raw material, such
as house waste, or compost at high temperatures with a controlled amount of oxygen
and/or steam. The resulting gas mixture is called synthesis gas or syngas and
is itself a fuel. Gasification is a method for extracting energy from many
different types of organic materials.
The
advantage of gasification is that using the syngas is potentially more
efficient than direct combustion of the original fuel because it can be
combusted at higher temperatures or even in fuel cells, so that the
thermodynamic upper limit to the efficiency defined by Carnot's rule is higher
or not applicable. Syngas may be burned directly in internal combustion
engines, used to produce methanol and hydrogen, or converted via the Fischer-Tropsch process into synthetic fuel. Gasification can
also begin with materials that are not otherwise useful fuels, such as biomass
or organic waste. In addition, the high-temperature combustion refines out
corrosive ash elements such as chloride and potassium, allowing clean gas
production from otherwise problematic fuels.
Gasification
of fossil fuels is currently widely used on industrial scales to generate
electricity. However, almost any type of organic material can be used as the
raw material for gasification, such as wood, biomass, or even plastic waste.
Gasification
relies on chemical processes at elevated temperatures >700°C, which
distinguishes it from biological processes such as anaerobic digestion that
produce biogas.
Gasification Chemistry
In
a gasifier, the carbonaceous material undergoes several different processes:
1. The pyrolysis (or devolatilization) process occurs as the carbonaceous
particle heats up. Volatiles are released and char is produced, resulting in up
to 70% weight loss for coal. The process is dependent on the properties of the
carbonaceous material and determines the structure and composition of the char,
which will then undergo gasification reactions.
2. The combustion process occurs as the
volatile products and some of the char reacts with oxygen to form carbon
dioxide and carbon monoxide, which provides heat for the subsequent gasification
reactions. Letting C represent a carbon-containing organic
compound, the basic reaction here is ![]()
3. The gasification process occurs as
the char reacts with carbon dioxide and steam to produce carbon monoxide and
hydrogen, via the reaction ![]()
4. In addition, the reversible gas phase water gas shift
reaction reaches equilibrium very fast at the temperatures in a gasifier. This
balances the concentrations of carbon monoxide, steam, carbon dioxide and
hydrogen. ![]()
In
essence, a limited amount of oxygen or air is introduced into the reactor to
allow some of the organic material to be "burned" to produce carbon
monoxide and energy, which drives a second reaction that converts further
organic material to hydrogen and additional carbon dioxide. Further reactions
occur when the formed carbon monoxide and residual water from the organic
material react to form methane and excess carbon dioxide. This third reaction
occurs more abundantly in reactors that increase the residence time of the
reactive gases and organic materials, as well as heat and pressure. Catalysts
are used in more sophisticated reactors to improve reaction rates, thus moving
the system closer to the reaction equilibrium for a fixed residence time.
History
The
gasification process was originally developed in the 1800s to produce town gas
for lighting and cooking. Electricity and natural gas later replaced town gas
for these applications, but the gasification process has been utilized for the
production of synthetic chemicals and fuels since the 1920s.
Wood
gas generators, called Gasogene or Gazogène, were used to power motor vehicles in
Europe during World War II fuel shortages.
Gasification processes
Four
types of gasifier are currently available for commercial use: counter-current
fixed bed, co-current fixed bed, fluidized bed and entrained flow.
The counter-current
fixed bed ("up draft") gasifier consists of a fixed bed of
carbonaceous fuel (e.g. coal or biomass) through which the "gasification
agent" (steam, oxygen and/or air) flows in counter-current configuration.
The ash is either removed dry or as a slag. The slagging gasifiers have a lower
ratio of steam to carbon, achieving temperatures higher than the ash fusion
temperature. The nature of the gasifier means that the fuel must have high
mechanical strength and must ideally be non-caking so that it will form a
permeable bed, although recent developments have reduced these restrictions to
some extent. The throughput for this type of gasifier is relatively low.
Thermal efficiency is high as the gas exit temperatures are relatively low.
However, this means that tar and methane production is significant at typical
operation temperatures, so product gas must be extensively cleaned before use.
The tar can be recycled to the reactor.
The co-current
fixed bed ("down draft") gasifier is similar to the
counter-current type, but the gasification agent gas flows in co-current
configuration with the fuel (downwards, hence the name "down draft
gasifier"). Heat needs to be added to the upper part of the bed, either by
combusting small amounts of the fuel or from external heat sources. The
produced gas leaves the gasifier at a high temperature, and most of this heat
is often transferred to the gasification agent added in the top of the bed,
resulting in an energy efficiency on level with the counter-current type. Since
all tars must pass through a hot bed of char in this configuration, tar levels
are much lower than the counter-current type.
In
the fluidized bed reactor, the fuel is fluidized in oxygen and
steam or air. The ash is removed dry or as heavy agglomerates that defluidize. The temperatures are relatively low in dry ash
gasifiers, so the fuel must be highly reactive; low-grade coals are
particularly suitable. The agglomerating gasifiers have slightly higher
temperatures, and are suitable for higher rank coals. Fuel throughput is higher
than for the fixed bed, but not as high as for the entrained flow gasifier. The
conversion efficiency can be rather low due to elutriation of carbonaceous
material. Recycle or subsequent combustion of solids can be used to increase
conversion. Fluidized bed gasifiers are most useful for fuels that form highly
corrosive ash that would damage the walls of slagging gasifiers. Biomass fuels
generally contain high levels of corrosive ash.
In
the entrained flow gasifier a dry pulverized solid, an
atomized liquid fuel or a fuel slurry is gasified with
oxygen (much less frequent: air) in co-current flow. The gasification reactions
take place in a dense cloud of very fine particles. Most coals are suitable for
this type of gasifier because of the high operating temperatures and because
the coal particles are well separated from one another. The high temperatures
and pressures also mean that a higher throughput can be achieved, however
thermal efficiency is somewhat lower as the gas must be cooled before it can be
cleaned with existing technology. The high temperatures also mean that tar and
methane are not present in the product gas; however the oxygen requirement is
higher than for the other types of gasifiers. All entrained flow gasifiers
remove the major part of the ash as a slag as the operating temperature is well
above the ash fusion temperature. A smaller fraction of the ash is produced
either as a very fine dry fly ash or as a black colored fly
ash slurry. Some fuels, in particular certain types of biomasses, can form slag
that is corrosive for ceramic inner walls that serve to protect the gasifier
outer wall. However some entrained bed type of gasifiers do not possess a
ceramic inner wall but have an inner water or steam cooled wall covered with
partially solidified slag. These types of gasifiers do not suffer from
corrosive slags. Some fuels have ashes with very high ash fusion temperatures.
In this case mostly limestone is mixed with the fuel prior to gasification.
Addition of a little limestone will usually suffice for the lowering the fusion
temperatures. The fuel particles must be much smaller than for other types of
gasifiers. This means the fuel must be pulverized, which requires somewhat more
energy than for the other types of gasifiers. By far the most energy
consumption related to entrained bed gasification is not the milling of the
fuel but the production of oxygen used for the gasification.
Current applications
In
small business and building applications, where the wood source is sustainable,
250-1000 kWe and new zero carbon biomass
gasification plants have been installed in Europe that produce tar free syngas
from wood and burn it in a reciprocation engines connected to a generator with
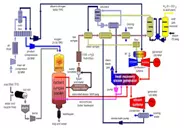
heat recovery. This type plant is often referred to as a wood biomass CHP unit
but is a plant of seven different processes: biomass processing, fuel delivery,
gasification, gas cleaning, waste disposal, electricity generation and heat
recovery.
Industrial-scale
gasification is currently mostly used to produce electricity from fossil fuels
such as coal, where the syngas is burned in a gas turbine.
Gasification
is also used industrially in the production of electricity, ammonia and liquid
fuels (oil) using Integrated Gasification Combined Cycles (IGCC), with the
possibility of producing methane and hydrogen for fuel cells. IGCC is also a
more efficient method of CO2 capture as compared to
conventional technologies. IGCC demonstration plants have been operating since
the early 1970s and some of the plants constructed in the 1990s are now
entering commercial service.
Gasification
technologies have been developed in recent years that use plastic-rich waste as
a feed.
Syngas
can be used for heat production and for generation of mechanical and electrical
power. Like other gaseous fuels, producer gas gives greater control over power
levels when compared to solid fuels, leading to more efficient and cleaner
operation.
Gasifiers
offer a flexible option for thermal applications, as they can be retrofitted
into existing gas fueled devices such as
ovens, furnaces, boilers, etc., where syngas may replace fossil fuels. Heating
values of syngas are generally around 4-10 MJ/m3.
Diesel
engines can be operated on dual fuel mode using producer gas. Diesel
substitution of over 80% at high loads and 70-80% under normal load variations
can easily be achieved. Spark ignition engines and SOFC fuel cells can operate
on 100% gasification gas. Mechanical energy from the engines may be used for
e.g. driving water pumps for irrigation or for coupling with an alternator for
electrical power generation.
Small-scale
rural biomass gasifiers have been applied in India to a large extent,
especially in the state of Tamil-Nadu in South India. Most of the applications
are 9 kWe systems used for water pumping
and street lighting operated by the local panchayat government. Although
technically applicable the systems face political, financial and maintenance
problems. Most of the systems are no longer running after 1–3 years.
While
small scale gasifiers have existed for well over 100 years, there have been few
sources to obtain a ready to use machine. Small scale devices are typically DIY
projects. However, currently in the US several companies offer gasifiers to
operate small engines.
Potential for renewable energy
In
principle, gasification can proceed from just about any organic material,
including biomass and plastic waste. The resulting syngas can be combusted.
Alternatively, if the syngas is clean enough, it may be used for power
production in gas engines, gas turbines or even fuel cells, or converted
efficiently to dimethyl ether (DME) by methanol dehydration, methane via the
Sabatier reaction, or diesel-like synthetic fuel via the Fischer-Tropschprocess. In many gasification processes most of the
inorganic components of the input material, such as metals and minerals, are
retained in the ash. In some gasification processes (slagging gasification)
this ash has the form of a glassy solid with low leaching properties, but the
net power production in slagging gasification is low (sometimes negative) and
costs are higher.
Regardless
of the final fuel form, gasification itself and subsequent processing neither
directly emits nor traps greenhouse gasses such as carbon dioxide. Power
consumption in the gasification and syngas conversion processes may be
significant though, and may indirectly cause CO2 emissions; in
slagging and plasma gasification, the electricity consumption may even exceed
any power production from the syngas. Combustion of syngas or derived fuels
emits the exact same amount of carbon dioxide as would have been emitted from
direct combustion of the initial fuel. Biomass gasification and combustion
could play a significant role in a renewable energy economy, because biomass
production removes the same amount of CO2 from the atmosphere
as is emitted from gasification and combustion. While other biofuel
technologies such as biogas and biodiesel are carbon neutral, gasification in
principle may run on a wider variety of input materials and can be used to
produce a wider variety of output fuels.
There
is at present very little industrial scale biomass gasification being done.
Examples of demonstration projects include those of the Renewable Energy
Network Austria, including a plant using dual fluidized bed gasification that
has supplied the town ofGüssing with 2 MW of
electricity and 4 MW of heat, generated from wood chips, since 2003.
Waste disposal
Several
gasification processes for thermal treatment of waste are under development as
an alternative to incineration.
Waste
gasification has several principal advantages over incineration:
● The necessary extensive flue gas cleaning may be
performed on the syngas instead of the much larger volume of flue gas after
combustion.
● Electric power may be generated in engines and gas
turbines, which are much cheaper and more efficient than the steam cycle used
in incineration. Even fuel cells may potentially be used, but these have rather
severe requirements regarding the purity of the gas.
● Chemical processing of the syngas may produce other
synthetic fuels instead of electricity.
● Some gasification processes treat ash containing heavy
metals at very high temperatures so that it is released in a glassy and
chemically stable form.
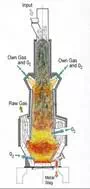
A
major challenge for waste gasification technologies is to reach an acceptable
(positive) gross electric efficiency. The high efficiency of converting syngas
to electric power is counteracted by significant power consumption in the
waste preprocessing, the consumption of large
amounts of pure oxygen (which is often used as gasification agent), and gas
cleaning. Another challenge becoming apparent when implementing the processes
in real life is to obtain long service intervals in the plants, so that it is
not necessary to close down the plant every few months for cleaning the reactor.
Several
waste gasification processes have been proposed, but few have yet been built
and tested, and only a handful have been implemented as plants processing real
waste, and always in combination with fossil fuels.
One
plant (in Chiba, Japan using the Thermoselect process)
has been processing industrial waste since year 2000, but has not yet
documented positive net energy production from the process.
Ze-gen is
operating a waste gasification demonstration facility in New Bedford,
Massachusetts. The facility was designed to demonstrate gasification of
specific non-MSW waste streams using liquid metal gasification.