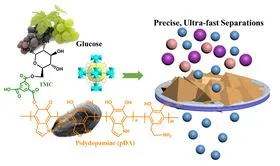
 ‘Green’ membrane fabrication process for sustainable membrane separations.
‘Green’ membrane fabrication process for sustainable membrane separations. ‘Green’ membrane fabrication process for sustainable membrane separations.
‘Green’ membrane fabrication process for sustainable membrane separations.
Removing contaminants like heavy metal ions, dye molecules, and salt compounds from water is essential for providing clean drinking supplies and treating industrial wastewater. Purification and recycling of organic solvents is also increasingly important to the pharmaceutical and petrochemical industries. Traditional purification and separation methods based on distillation, where liquids are boiled off to leave solid waste behind, are expensive and energy intensive. Low-energy alternatives rely on advanced separation techniques, where contaminated liquids are forced through a membrane to remove unwanted material. But the fossil-fuel-derived raw materials needed to synthesize these membranes undermine the energy benefits of the approach.
Now researchers from China, UK, and USA have created ‘green’ membranes based on natural materials that surpass state-of-the-art membranes for desalination, cleaning up wastewater, and purifying/recycling organic solvents
“State-of-the-art polymer membranes are typically produced from synthetic materials via complex protocols and material synthesis procedures, while natural materials are rarely employed due to their intrinsically low separation performances and poor chemical stability,” explains Lu Shao of Harbin Institute of Technology in China, who led the study.
To avoid using polymers derived from fossil fuels, Shao and colleagues from Lawrence Berkeley National Laboratory and The University of Edinburgh turned to natural alternatives. The new membranes consist of glucose-based saccharides (or sugar molecules) and mussel-inspired synthetic polymers known as polydopamines deposited onto porous membrane substrates in combination with zirconium-based metal organic frameworks (MOFs) in which metal ions are held together by organic linker molecules to form highly porous structures.
The ultrathin nanocomposite membranes allow fast, efficient, and low-pressure separation of contaminants from water and organic solvents. In tests, the new membranes outperformed other MOF-based nanocomposite membranes and commercially available nanofiltration membranes in the removal of ion/molecule compounds from contaminated liquids. The membranes were also highly stable during water or organic solvent filtration, showing no signs of degradation after 240 hours of testing.
“In addition, the membrane… can reduce the energy consumption under lower operational pressures and possesses… good anti-fouling performance,” points out Shao.
The new membranes could be used for both traditional water purification and solvent treatment in diverse industries from pharmaceuticals to petrochemicals. Different polysaccharides could be used in combination with other nanoporous additives to optimize the performance, suggest the researchers.
“As fossil fuel supplies are fast depleting, there is a need to identify alternative natural resources for next-generation membranes,” says Shao. “More importantly, the low biodegradability of synthetic polymers is a major cause of pollution and other environmental issues. Our approach provides a new synthesis platform to realize next-generation membranes with inherent natural properties and unparalleled performance… in ultra-fast, low-pressure, precise separations, even in organic solvent systems.”