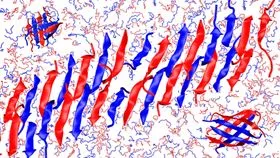

This image is an adaptation of a computer simulation of the CATCH(+) and CATCH(-) mixture of peptides.
A team of researchers has verified that it is possible to engineer two-layered nanofibers consisting of an ordered row of alternating peptides, and has also determined what makes these peptides automatically assemble into this pattern. This fundamental discovery raises the possibility of creating tailored ‘ABAB’ peptide nanofibers for a variety of biomedical applications.
Peptides are small proteins, made up of short strands of amino acids. It's well established that peptides can self-assemble into nanofibers composed of beta-sheets, but that self-assembly normally involves identical copies of the same molecule – molecule A connects to another molecule A.
This new work, reported in a paper in the Proceedings of the National Academy of Sciences, proves not only that alternating peptides can create these beta sheets – in an ABAB pattern – but why it happens.
"Our team drew on computational simulations, nuclear magnetic resonance (NMR) observations and experimental approaches for this work, and we now know what drives the creation of these alternating peptide structures," says Carol Hall, professor of chemical and biomolecular engineering at North Carolina State University and corresponding author of the paper. "This is important because once you understand why peptides in these ABAB structures are behaving in this way, you can develop more of them."
For this study, researchers worked with a pair of peptides called CATCH(+) and CATCH(-). When introduced into a solution, these peptides array themselves in a row, alternating the two peptides. The peptides also assemble in two beta-sheet layers per nanofiber.
The study involved three components. Greg Hudalla's lab at the University of Florida created the peptides, facilitated the co-assembly of the peptide beta sheets and performed experimental work that provided an overview of the system and its behavior. Meanwhile, Anant Paravastu's team at Georgia Tech used solid-state NMR to measure the precise relative positions of atoms and molecules in the ABAB peptide beta-sheets. Lastly, Hall's team at North Carolina State University conducted computational simulations to determine what was driving the behavior seen by the researchers at the University of Florida and Georgia Tech.
There appear to be multiple forces at play in guiding the assembly of the alternating peptide structures. One of the two types of peptide is negatively charged, while the other type is positively charged. Because positive and negative attract each other, while peptides of the same charge repel each other, this leads to the alternating order of peptides in the strand.
Another aspect of the system's organization, the stacking, is driven by the types of amino acids in each peptide. Specifically, some of the amino acids in each peptide are hydrophobic, while others are hydrophilic. The hydrophobic amino acids, in effect, want to stick to each other, which results in the two-layer ‘stacking’ effect seen in the beta-sheets.
"It is important that different forces balance to produce the target structure," Hall says. "If any one of the molecular forces is too strong or too weak, the molecules may never dissolve in water or may fail to recognize their intended partners. Rather than an ordered nanostructure, the molecules could form a disorganized mess, or no structure at all."
"We're interested in this because it gives us a glimpse into the fundamental nature of how these systems can work," Hudalla says. "We're not aware of any similar co-assembling systems in nature that resemble the system we've made here.
"Co-assembling peptide systems hold promise for biomedical applications because we can attach proteins to the A or B peptides that have some specific utility. For example, we could create a peptide scaffold that holds a regular array of enzymes, and those enzymes could serve as catalysts for influencing body chemistry in localized areas."
"The structures we're making here are impressive, but they are still not as precise and complex as biological structures that we see in nature," Paravastu says. "By the same token, we're not aware of natural structures that contain this alternating peptide structure. This is a good start. We are excited to see where it goes."