Forms of Heat Energy Ė Latent
If you took high school chemistry, you learned that water is created when two gases, hydrogen and oxygen are combined. You may have even been lucky enough to have a teacher who was able to perform this magical transformation live during class.
Depending primarily on the amount of heat energy absorbed, water exists in one of the three states of matter, gas, liquid, or solid. Its states also depend on surrounding atmospheric pressure, but more about that later. For our discussion, the water will reside at the atmospheric pressure present at sea level, which is around 14.7 pounds per square inch.
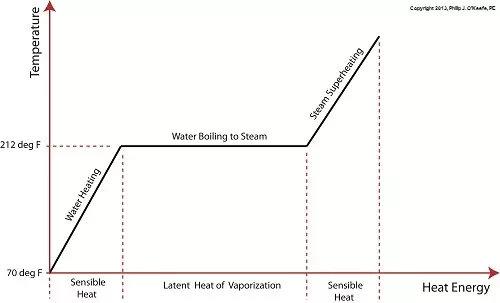
Last time we learned that the heat energy absorbed by water before it begins to boil inside our example tea kettle is known as sensible heat within the field of thermodynamics. The more sensible heat thatís applied, the more the water temperature rises, but only up to a point.
The boiling point of water is 212įF. In fact this is the maximum temperature it will achieve, no matter how much heat energy is applied to it. Thatís because once this temperature is reached water begins to change its state of matter so that it becomes steam. At this point the energy absorbed by the water is said to become the latent heat of vaporization, that is, the energy absorbed by the water becomes latent, or masked to the naked eye, because it is working behind the scenes to transform the water into steam.
As the water in a tea kettle is transformed into steam, it expands and escapes through the spout, producing that familiar shrill whistle. But what if we prevented the steam from dispersing into the environment and continued to add heat energy? Ironically enough, under these conditions temperature would continue to rise, upwards of 1500įF, if the stoveís burner were powerful enough. This process is known as superheating. Now hold your hats on, because even more ironically, the heat added to this superheated steam is also said to be sensible heat.
Confused? Letís take a look at the graph below to clear things up.
Sensible heat is heat energy thatís added to water, H2O, in its liquid state. Itís also the term used to describe the heat energy added to steam thatís held within a captive environment, such as takes place during superheating. On the other hand, the latent heat of vaporization, that is the heat energy thatís applied to water once itís reached boiling point, does not lead to a further rise in temperature, as least as measured by a thermometer.