MELAMINE-FORMALDEHYDE RESINS
PROPERTIES
Melamine formaldehyde (also called melamine or MF) is a hard, very durable, and versatile thermosetting aminoplast1 with good fire and heat resistance. It is made from melamine and formaldehyde by condensation of the two monomers. Its good fire retardant properties are due to the release of nitrogen gas when burned or charred.
MF resins are quite similar to urea-formaldehyde (UF) resins and are fully compatible with them and, therefore, are often co-reacted with them to reduce formaldehyde emissions from particleboards and to prevent degradation of glue bonds caused by hydrolytic degradation of the UF polymers. The resin blend is called melamine–urea–formaldehyde (MUF). Compared to natural woods or wood veneers, melamine composite boards and overlay materials have improved heat, moisture, scratch, and chemicals resistance.
Melamine can also be converted to foam structures with a distinctive pore structure, which is extremely hard. It can be used as an insulating and soundproofing material and more recently as a cleaning abrasive.
MF resins compete in many applications with urea-formaldehyde (UF) and phenolic resins (phenoplasts). In general, MF resins are harder and stronger than phenolics but have lower moisture and heat resistance. Unlike phenolics, they are clear and colorless. Thus, MF resin can be molded into products of light color. Compared to UF resins, MF resins have somwhat better properties including better moisture and heat resistance, but they are also more expensive.
SYNTHESIS
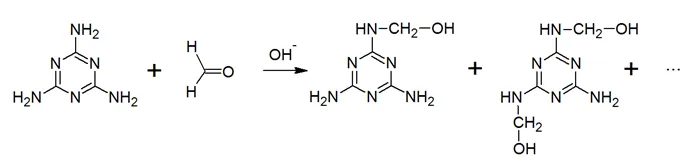
Melamine formaldehyde (MF) resins are primarily made up of melamine and formaldehyde with formaldehyde acting as the crosslinker. The melamine reacts with formaldehyde under slightly alkaline conditions to form mixturers of various methylolmelamines:

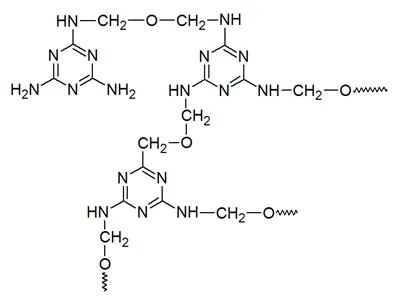
Further heating causes condensation of the methylolmelamines:
The monomeric form hexamethylolmelamine and the MF resins are very versatile crosslinking agents for a wide variety of polymeric materials both water- and solventborne. To improve the solubility in solvents, melamine formaldehyde (MF) resins, and the monomeric forms are often etherified with alkohols such as methanol and n-butanol. After acidification, they readily react with thiol, hydroxyl, carboxyl and amide groups to form three-dimensional thermoset polymer networks. The relative reaction rate for these functional groups follows the order of - SH > - OH > - CONH2 > - COOH.
In the case of the not etherified compounds, acidification is not necessary and heating alone is often sufficient for network formation.
APPLICATIONS
Melamine resins are used for the manufacture of many products, including kitchenware, laminates, overlay materials, particleboards, and floor tiles. Melamine and its salts are also used as fire-retardant additives in paints, plastics, and paper.
Melamine foam is a special form of melamine resin. It finds uses as an insulating and soundproofing material and more recently as a cleaning abrasive. The foam products can be used for removing scuffs and dirt from a wide range of surfaces. Furthermore, some filters are made out of porous melamine. These filters can be used in hot environments, and are extremely efficient.
Melamine is or has been used in the manufacture of flame-resistant fibers and textiles. These include upholstery, firemen uniforms, thermal liners, and heat resistant gloves and aprons.
Sulfonated melamine formaldehyde (SMF) is used as a super-plasticizer for making high-resistance concrete. It is mainly added to reduce the water content in concrete while increasing the fluidity and the workability of it during handling and pouring. The result is a concrete with a lower porosity and a higher mechanical strength, which exhibits an improved resistance to aggressive environments and has, therefore, a longer lifetime.
One of the most important applications of melamine is the fabrication of particleboards. It is often blended with UF resins to improve the scratch and flame resistance and also acts as a flame retardant. Melamine is also used to saturate decorative paper, which is then laminated under heat and pressure, fusing the laminate onto particleboards. The melamine composite boards and laminates are a cost effective alternative to more expensive surfaces. The main advantage is reduced material costs, and quick turn-around times, resulting in reduced labor costs.
(Etherified) hexamethylolmelamine and pure melamine (2,4,6-triamino-1,3,5-triazine) are important crosslinkers for many industrial thermosetting coatings. They readily react with primary and secondary hydroxyl functional polymers, resulting in three-dimensional thermoset polymer networks. They are used to crosslink alkyds, epoxies, and polyester polyol resins. There are many types of melamine surface coatings that cover a broad range of properties and cure speeds varying from very slow to very fast cure.