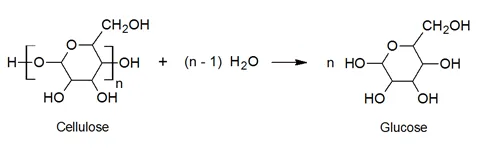
CELLULOSE AND ITS DERIVATIVES
PROPERTIES
Cellulose is the most abundant polysaccharide found in nature. It is a linear polymer consisting of 6-member ether rings (D-glucose or dextrose) linked together covalently by ether groups, the so-called glycosidic bonds. Usually many thousand glucose repeat units make up a cellulose polymer. Cellulose and its derivatives can be considered condensation polymers because their hydrolysis yields glucose molecules:
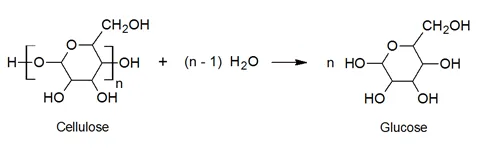
The cyclic structure in the main polymer chain together with strong hydrogen bonding gives cellulose a rigid structure. Thus, cellulose and some of its derivatives have a high glass transition temperature and melting point. The strong intermolecular hydrogen bonds between the hydroxyl groups lead to highly ordered crystalline regions with low accessibility to reactants which explains why cellulose is water insoluble and why strong alkalis like caustic soda are required to break down the structure to make the hydroxyl groups accessible to reactants.
Cellulose is the most abundant organic polymer on the planet. It is an important structural component of the primary cell wall of plants. The cellulose content of cotton fibers is about 90 percent. Not surprising, it is the main raw material for many semi-synthetic cellulose derivatives.1
The most important cellulose esters are cellulose acetate (CAc), and the co-esters cellulose acetate-propionate (CAP), and cellulose acetate-butyrate (CAB). Among these, cellulose acetate is by far the most important cellulose ester. It was first used for photographic film and later as a coating for fabric on airplanes. Like cellophane it is made from cellulose but has very different properties. Unlike cellophane, it is thermoplastic, that is, it will soften and melt when heated.
The most common source of cellulose is cotton linters. The fibers are mixed with glacial acetic acid and acetic anhydride with sulfuric acid as a catalyst. This results in cellulose triacetate. In a subsequent step water is added to stop the reaction and to partially hydrolyze the triacetate:
Cellulose acetate is a crystal clear, tough, and flexible plastic and is the most stable cellulose derivative. It has excellent chemical resistance to organic and inorganic weak acids, hydrocarbons, vegetable oils, and the like. Often plasticizers are added to further increase its flexibility or mixed ester of cellulose like butyrate-acetate and propionate-acetate are produced which have improved flexibility, toughness, and moisture resistance.
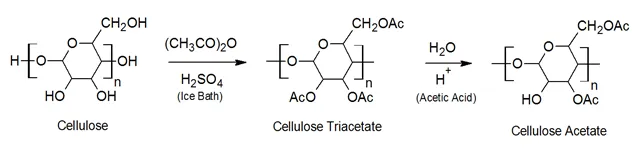
Cellulose ethers are produced from wood pulp or cotton linters. The cellulose is treated with a solution of sodium hydroxide in a process similar to cellophane. In a subsequent step, the alkali cellulose is treated with an alkyl halide or an epoxide. The first method is frequently used to prepare ethyl celulose whereas the second method is used to prepare and hydroxethyl and hydroxpropyl cellulose. Alternatively, the alkali cellulose can be treated with alkyl sulfate. For example, methyl sulfate treatment is a common process for the manufacture of methyl cellulose.
The most important modified cellulose polymers are methyl cellulose (MC), and ethyl cellulose (EC).
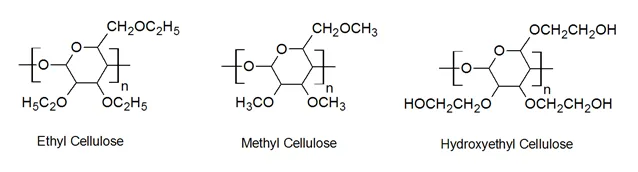
Other commercially important cellulose ethers include hydroxylpropylcellulose (HPC), hydroxyethylcellulose (HEC), and carboxymethylcellulose CMC. These polymers can be produced by treating alkali celulose with epoxides (HPC, HEC) or with chloroacetate (CMC).
Methyl cellulose (MC) is the most important commercial cellulose ether. It is also the simplest derivative where methoxy groups have replaced the hydroxyl groups. The most important properties of this nonionic polymer are its water solubility and its gelation when exposed to heat. Although soluble in water, films made from methyl cellulose usually retain their strength and do not become tacky when exposed to humidity. Polymer films made of methyl cellulose have excellent strength (60 - 70 MPa) and low elongation (5 - 15 %) at room temperature (75°F) but its strength decreases rapidly with increasing temperature. MC also has excellent UV, oil, and solvent resistance.
Ethyl cellulose (EC) is another important commercial cellulose ether derivative. While complete etherification is possible yielding triethyl cellulose usually only to 2 to 2.5 ethoxyl groups per glucose unit are etherified. This polymer has excellent strength at room temperature but its strength decreases rapidly with increasing temperature.
The lower the number of ether groups the greater will be the toughness and the lower the solubility, but poorer compatibility with plasticizers and other additives will result.
Partially hydrolyzed cellulose ethers and esters can also be converted to thermoset resins. The crosslinking can be achieved by reacting the residual hydroxyl groups with urea formaldehydes, melamines, or polyisocyantes.
Nitrocellulose (NC), also called cellulose nitrate, is the oldest thermoplastic. It was invented by Alexander Parkes in 1855 and later commercialized under the trademarks Parkesine, Xylonite and Celluloid. To achieve the desired properties, other additives such as camphor, dyes, stabilizers and fillers are added.
Cellulose nitrate itself is synthesized by mixing cellulose fibers with an aqueuos solution of nitric and sulfuric acid. The fibers are immersed in this solution for 20 to 60 minutes at 30 to 40°C. The product is then repeatedly washed with water and sodium carbonate solution to neutralized and remove the acids.
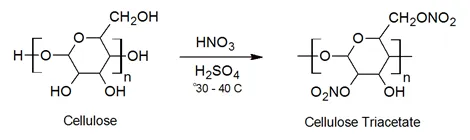
The average degree of nitration will be affected by the water content, composition of the bath, immersion time, and reaction condition. NC's with about 2 nitrate groups per glucose repeat unit are often chosen in plastics and laquers. A higher nitrate content is used in explosives.
Cellulose nitrate has excellent mechanical properties. However, plastics made from NC like celluloid have poor weathering and heat resistance and are not resistant to dilute acids and bases, but are insoluble and stable in water and nonpolar solvents.
Nitrocellulose is highly combustable which makes it too hazardous for most applications.2 Today, NC is mainly used as a binder in products like inks, coatings, and adhesives. The dilution with other ingredients greatly reduces its flammability.