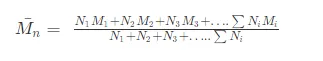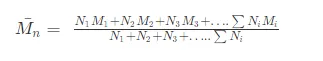
Types of Polymerization Reactions
Addition Polymerization
This is also called as chain growth polymerization. In this, small monomer units joined to form a giant polymer. In each step length of chain increases. For example, Polymerization of ethane in the presence of Peroxides
Condensation Polymerization
In this type small molecules like H2O, CO, NH3 are eliminated during polymerization (step growth polymerization). Generally, organic compounds containing bifunctional groups such as idols, -dials, diamines, dicarboxylic acids undergo this type of polymerization reaction. For example, Preparation of nylon -6, 6.
What is Copolymerization?
In this process, two different monomers joined to form a polymer. Synthetic rubbers are prepared by this polymerization. For example, BUNA – S.
How to Calculate Molecular Mass of Polymers?
There are two types of average molecular masses of Polymers:
Number Average Molecular Masses:
If N1, N2, N3…. are the number of macromolecular with molecular masses. M1, M2, M3….. Respectively then the number average molecular masses of the polymer is given by