FLORY-HUGGINS PARAMETER
Many thermodynamic properties of polymer solutions such as solubility, phase equlibria as well as swelling equilibria of isolated polymer coils and polymer networks are often expressed in terms of the polymer-solvent interaction parameter. This parameter was first introduced by Flory1 and Huggins2 independently to describe the interaction between solvent and polymer molecules in their lattice model of polymer solutions.

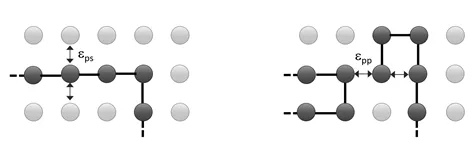
To calculate this parameter, we assume that the polymer segments and the solvent molecules are randomly distributed in the coil volume R3, and that the heat of mixing is proportional to the volume fraction of polymer segments in the coil volume, R3. The overall system energy is the sum of all interactions between neighboring molecules. For simplicity, we further assume that the solvent and polymer segments are of same size and that only nearest neighbor interaction have to be included in the calculation. For this simple case, the energy if mixing can be written as:
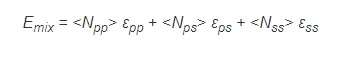
where ![]() is the interaction energy between two molecules, and <Nij> is the average number of contacts between molecules i and j. The probability Pv that a site is occupied by a molecule of type i is simply proportional to its volume fraction:
is the interaction energy between two molecules, and <Nij> is the average number of contacts between molecules i and j. The probability Pv that a site is occupied by a molecule of type i is simply proportional to its volume fraction:
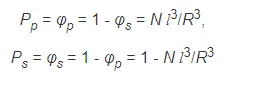
where l is the effective length of a monomer. Hence, the average number of solvent-polymer, polymer-polymer and solvent-solvent pairs is given by
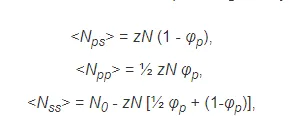
where N is the number of repeat units (segments) in the polymer chain, N0 is the pairs of neighboring solvent molecules in the absence of a polymer, and z is the (average) number of contacts per molecule, also called lattice coordination number (Flory-Huggins lattice model). Inserting the three expressions for the pair contacts into the equation for the energy of mixing gives
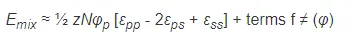
Flory defined a dimensionless quantity which characterizes the interaction energy per solvent molecule divided by kT:
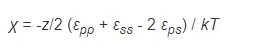
This quantity is called polymer-solvent interaction parameter or Flory-Huggins parameter because it was first introduced by P. J. Flory1 and M. L. Huggins2 as an exchange interaction parameter in their lattice model of polymer solutions. zkTχ is simply the difference in energy between a solvent molecule immersed in pure polymer and in pure solvent. If we introduce this parameter, the energy of mixing can be written as
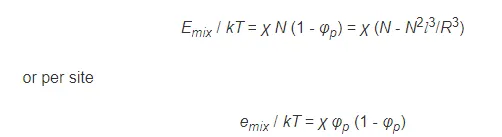
Thus, the effect of solvent interactions can be expressed in terms of a single parameter.