EMULSION POLYMERIZATION
Emulsion polymerization is one of the most important methods for the polymerization of a large number of monomers, like vinyl acetate, vinyl chloride, chloroprene, acrylamide, acrylates, and methacrylates. It is also used for the production of various copolymers, like acrylonitrile-butadiene-styrene (ABS).
Emulsion polymerization has several advantages over other polymerization techniques; for example, it is more rapid than bulk or solution polymerization at the same temperature, the conversion is essentially 100 percent, and the average molecular weight is usually (much) higher than at the same polymerization rate in bulk or solution polymerization. Also, heat dissipation and viscosity control are much less problematic than in bulk polymerization.
In general, an emulsion polymerization system consists of a dispersing medium, monomer, emulsifier, initiator and, if necessary, modifiers. Water1 is normally the continuous phase in which the various components are dispersed by the emulsifiers. The monomers are only slightly soluble in water.4 They form droplets that are suspended and stabilized by the emulsifiers, that is, the emulsifier molecules associate and form micelles that surround small amounts of monomer. The remaining monomer is dispersed in small droplets.
Common emulsifiers are anionic and nonionic surfactants whereas cationic surfactants such as quaternary ammonium salts are rarely used. Typical anionic emulsifiers are sodium, potassium, or ammonium salts of fatty acids and C12 - C16 alkyl sulfates. Typical nonionic surfactants are poly(ethylene oxide), poly(vinyl alcohol) and hydroxyethyl cellulose. A combination of both anionic and nonionic surfactants will often improve the stability of the dispersed droplets.
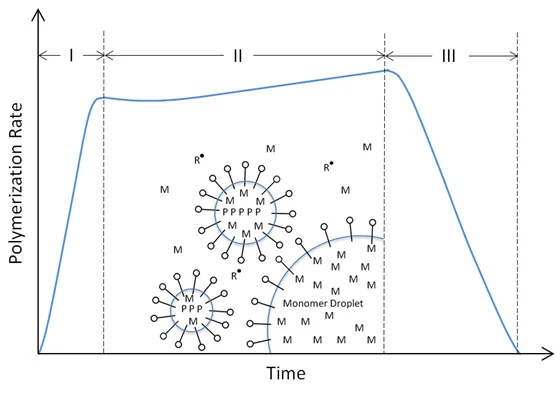
The first hypothesis of the mechanism of emulsion polymerization was proposed by Harkins2; the process can be divided in three distinctive stages:
STAGES OF AN EMULSION POLYMERIZATION

At the beginning, the mixture consists solely of the continuous water phase with dispersed surfactant micelles3 and emulsified monomer droplets of 1-10 microns. Most monomer is localized in these droplets and some is dissolved in the micelles and a little in the water phase. The micelles are in a dynamic equilibrium with the dissolved emulsifier molecules. Nucleation stops when the surface area becomes large enough to absorb all of the emulsifier molecules. The polymerization starts when initiator is added. A common initiator is (water soluble) potassium persulfate.5 It decomposes in the water and forms negatively charged sulfate radicals:
S2O8- + Heat → 2 SO4·-
These radicals react with the in the water dissolved monomers and form soap-type free radicals.
SO4·- + (n+1) M → SO4-−(CH2-CX2)n−CH2-CX2·
They either associate with the dissolved emulsifier molecules to micelles or they migrate into existing micelle droplets. During the first stage of the polymerization, monomers continuously migrate from the large monomer droplets through the water phase into the micelles and are added to the growing polymer chains. At the same time, new particles are formed by the initiators. Some polymerization takes also place in the water phase due to the solubility of the monomers and initiators, whereas the monomer droplets do not provide loci for polymerization because the negatively charged surfactant molecules that surround these droplets are virtually impossible to penetrate by the also negatively charged initiator molecules.
The number of polymer particles and the rate of polymerization increase as long as new radicals and polymer micelles are formed. Eventually, all surfactant in the system has been absorbed or all initiator molecules have been used up by the polymer particles. At this point in time, the rate of polymerization remains more or less constant. The particle number stabilizes at a rather low value which is only a small fraction, typically about 0.1% of the number of micelles initially present.
The diffusion of the free radicals into the monomer droplets is generally not important to the reaction rate during the growth stage and the rate of monomer diffusion is usually adequate to supply enough monomer to keep the reaction in the particles going.
As the size of the latex particles increases, the size of the monomer droplets decreases and eventually they disappear. At this stage, the reaction mixture consists solely of monomer swollen polymer particles, the so-called latex particles, and dissolved monomers. Since monomer droplets are no longer present, both the monomer concentration and the reaction rate steadily decrease with time. With increasing polymer concentration, some cross-linking reactions and branching can also be expected. The reaction ends when either all monomers are used up or when a second radical diffuses into the polymer particles and causes immediate bimolecular termination by disproportionation or recombination of two radicals. If no termination occurs, the system reaches a conversion of essentially 100 percent. The final (submicroscopic) polymer particles are spherical in shape and have a diameter in the range of 50 – 300 nm which is between the initial micelle and monomer droplet size.