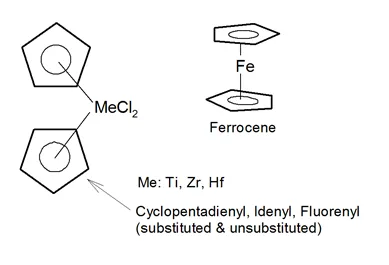
METALLOCENE COORDINATION CATALYST
Metallocenes are very effective metal catalyst. They consist of minute particles of positively charged metal ions sandwiched between two cyclopentadienyl anions, which have five atoms per ring. They are also known as single-site catalysts because they have only one active site per catalyst particle, which are all identical. Metallocene catalysts were discovered in the 1950s.1 Despite the early discovery, it took more than 20 years before technology was developed that allowed the ecconomicall large scale production of metallocene polyolefins. A significant breakthrough was achieved in the late ’70s when Sinn and Kaminsky combined metallocene catalysts with methylalumoxane [(MeAlO)n] (MAO) which enhances the activity by a factor of 10 000.2-4
The metallocene catalyzed polymerization has many advantages over traditional polymerization techniques; it results in very pure, and consistent resins with well defined properties. For example, a wide variety of metallocene polyethylenes can be produced with very different properties ranging from very soft with low melting point to high melting point with good heat resistance. Many derivatives of early metallocenes are active catalysts for olefin polymerization. Unlike traditional and still widely used heterogeneous Ziegler-Natta catalysts, metallocenes are homogeneous. The polymerization with metallocenes and other coordination catalyst involves coordination of the monomer to the transition metal site before insertion at one end of the polymer chain. The coordination step is responsible for the versatility of these catalysts; since the arriving monomer needs to coordinate to the active site before propagation can occur, the electronic and steric environment around it will control the polymerization and, thus, the polymer microstructure, the propagation and chain transfer rates, the co-monomer reactivity ratios, and the stereo- and regio-selectivity.
Only a few metallocene anions are known. The most famous example is ferrocenium, [Fe(C5H5)2]+, which is the blue iron-III-complex derived from oxidation of orange iron-II-ferrocene. Other important five member rings are idenyl and fluorenyl.
