FREE RADICAL POLYMERIZATION
Free radical polymerization (FRP) is one of the most important synthesis routes for obtaining vinyl polymers. The relatively non-specific nature of the free radicals towards vinyl (unsaturated) monomers makes it one of the most versatile polymerization methods. Almost 40% of all polymers produced in the United States are produced by this mechanism. This includes polystyrene, poly(methyl methacrylate), poly(vinyl acetate) and polyethylene, and many other large volume polymers.
A common feature of the vinyl polymerization is that the active center of the growing chain is retained by a single polymer molecule throughout the course of its growth. Thus the partially polymerized mixture consists of high molecular weight polymers and unchanged monomers with virtually no chains of intermediate molecular weight. In fact, polymers formed during the early stage of polymerization, even during the first fraction of a percentage conversion, are comparable in molecular weight to those formed at the advanced stage of the process.
Most vinyl monomers are readily susceptible to catalysts and photo-activation. For this reason, inhibitors are usually added to the monomer to prevent uncontrolled polymerization. The efficiency of certain inhibitors is of the order 103 or higher, thus a single molecule of inhibitor prevents thousands of monomer molecules from polymerizing.
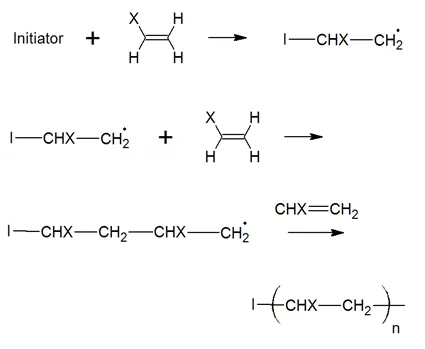
The polymerization starts off with a molecule called initiator. Common initiators are benzoyl peroxide (BPO) and 2,2'-azo-bis-isobutyrylnitrile (AIBN). Both molecules have a strong tendency to fall apart in a rather unusual way; that is, when they split, the pair of electrons in the bond which is broken, will separate. The two fragments with unpaired electrons are called free radical initiators. Following its generation, the initiating free radicals react with a monomer unit thereby creating growing polymer chains:

where X is a substituent which could be any of the following: C6H5, Cl, Br, OCOCH3, COOR or H. The mechanism also includes disubstituted monomers such as vinylidene chloride and methyl methacrylate.