TOXIC ADDITIVES
A significant challenge to the recycling of plastic is the widespread use of chemical additives in the formulations of polymers, many of which are inherently toxic. The recipe of additives in a plastic depends on the intended application of the polymer. Industry analysts estimate that the global plastic additives market for plasticizers, flame retardants, antioxidants, antimicrobial, UV stabilizers, and blowing agents will grow from USD 43.82 billion in 2018 to USD 61.25 billion by 2025 (Zion 2020). While some of the inert additives such as clays and talc are relatively benign, many additives are toxic to humans as well as environmental pollutants.
Additives can be divided into four main categories:
· Functional additives (stabilizers, antistatic agents, flame retardants, plasticizers, lubricants, slip agents, curing agents, foaming agents, biocides, etc.)
· Colorants (pigments, soluble azocolorants, etc.)
· Fillers (mica, talc, kaolin, clay, calcium carbonate, barium sulphate)
· Reinforcements (e.g. glass fibres, carbon fibres).
The most commonly used additives in different types of polymeric packaging materials are plasticizers, flame retardants, antioxidants, acid scavengers, light and heat stabilizers, lubricants, pigments, antistatic agents, slip compounds, and thermal stabilizers (Hahladakis et al., 2018). Of the functional additives, plasticizers, stabilizers and flame retardants tend be of most concern to human health, but some colorants and fillers can also be problematic. Some of the most problematic functional additives are described below.
Flame retardant additives can be extremely toxic and have a high concentration by weight in the polymer, most especially among older waste electrical and electronic equipment (WEEE) plastics and automotive plastics. These additives commonly contain bromine compounds and are known as brominated flame retardants (BFRs). Plastics containing BFRs are produced in high volumes and have flame retardants added to reduce their flammability. In automobiles the plastics in dashboards, seating and
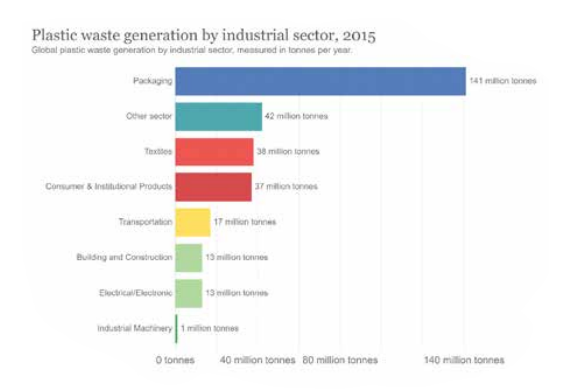
Figure 4. Plastic waste generation by industrial sector in 2015.
upholstery are often heavily treated with flame retardants with the intention of reducing the risk of fires. In electronics, the casings of TVs, computers and wire insulations are often treated with flame retardants due to the risk of fire from electrical short circuits etc. Some BFRs have been assessed as POPs and have been banned under the Stockholm Convention (e.g. Polybrominated dipheyl ethers (PBDE), Hexabromocyclododecane (HBCD or HBCDD)). These brominated POPs are known as POP-BFRs. Other brominated flame retardants may still be hazardous but have not been assessed as POPs.
Even after these additives have been banned, they continue to be present in plastic waste stockpiles for many years as the products they were used in may take years or decades to reach their end of life and enter the waste stream. Plastics containing POPs BFRs can generate dangerous exposure for recycling workers, including releases of brominated dioxins at the remelting and extrusion phase . Unless carefully separated from polymers that are not contaminated by POP BFRs, these POPs can leak into the overall polymer recycling chain, ending up in products such as toys and kitchen utensils that increase human exposure, especially among children ,as well as in food contact materials and household products. Not all flame retardants are brominated. Short, medium, and long-chained chlorinated paraffins (SCCPs/MCCPs/LCCPs) have also been used extensively in plastics as both a plasticizer and flame retardant. Some flame retardants are so toxic and persistent that they have been identified as POPs and made subject to elimination through the Stockholm Convention. Specifically, Hexabromobiphenyl (HBB), commercial PentaBDE, commercial OctaBDE, commercial DecaBDE, Hexabromocyclododecane (HBCD), and short-chained chlorinated paraffins (SCCPs) have been added to the annexes of the convention as POPs.
Plasticizer additives also have a significant potential to impact on human health. Around 80% of all plasticizers are used in polyvinyl chloride (PVC) plastics and include short, medium, and long-chain chlorinated paraffins (SCCP/MCCP/LCCP), Diisoheptyl phthalate (DIHP), 1,2-Benzenedicarboxylic acid, di-C7-11 -branched and linear alkyl esters (DHNUP), Benzyl butyl phthalate (BBP), Bis(2-ethylhexyl) phthalate (DEHP), Bis(2-methoxyethyl) phthalate (DMEP), Dibutyl phthalate (DBP), dipentyl phthalate (DPP), di-(2-ethylhexyl) adipate (DEHA), di-octyladipate (DOA), diethyl phthalates (DEP), diisobutyl phthalate (DiBP), Tris(2 chloroethyl) phosphate (TCEP), dicyclohexyl phthalate (DCHP), butyl benzyl phthalate (BBP), diheptyl adipate (DHA), heptyl adipate (HAD), and heptyl octyl adipate (HOA), while the remaining 20% are used in cellulose plastic .
The plasticizer group of additives are dominated by phthalates, which can have significant health impacts when they leach or migrate out of the polymer structure. They can also have impacts when the plastic reaches its end of life, degrades in the environment, leaches into landfill, or is burned. Phthalates are a known endocrine disrupting chemical (EDC) capable of disrupting the hormonal system of humans. This means they can have farreaching and devastating impacts on the fetus through childhood development and into adulthood.3 EDCs can also have serious impacts on the ability of wildlife and aquatic organisms to develop and on their ability to reproduce. Plasticizers are commonly used in flexible food packaging and food contact materials as additives to the polymer packaging. Phthalates are used in large volumes in PVC.
EDCs and their impacts on human health are a growing area of scientific research. Their impacts can be unpredictable and difficult to manage, according to existing regulatory frameworks such as linear dose-response relationships (i.e. that the dose makes the poison) as assessed by risk assessment frameworks. EDCs can impact human health at very high levels but also at very low levels, especially during the early development of the fetus and other sensitive developmental windows. The ability to impact health in a non-linear dose relationship defies established threshold-level assessments used to predict the potential harm of a chemical. As noted in a 2014 Endocrine Society/IPEN publication, a positive correlation between chemical and plastic production and EDC effects in humans is evident:
“There is good reason to suspect that increasing chemical production and use is related to the growing incidence of endocrine-associated pediatric disorders over the past 20 years, including male reproductive problems (cryptorchidism, hypospadias, testicular cancer), early female puberty, leukemia, brain cancer, and neurobehavioral disorders. At the same time, the global production of plastics grew from 50 million tons in the mid-1970s to nearly 300 million today, and sales for the global chemical industry have sharply increased from USD $171 billion in 1970 to over USD $4 trillion in 2013. Chemicals such as polychlorinated biphenyls (PCBs), BPA, and phthalates, are now detectable in serum, fat, and umbilical cord blood in humans around the globe.”
There are many routes by which EDCs additives can leave plastics and cause exposure to humans. For instance, Bisphenol-A (BPA), is a very commonly used plasticizer and another known EDC that has been reported to migrate into food from plastic baby bottles , heat-affected food can coatings , and other types of food packaging . Many food packaging containers are now produced ‘BPA-free’, but BPA is a highvolume chemical and common in plastics so exposure can continue from a variety of sources.
Toxic additives from plastic enter the environment in a range of ways through emissions, releases, degradation, leaching, and migration (Figure 5) at many stages during the production, use, and end of life of the plastic they have been added to. The propensity of additives to leave the polymer is increased by the fact they are not chemically (covalently) ‘bound’ in the polymeric chain but rather are dispersed between monomers (Figure 6). This allows a greater propensity to migrate out of the polymer into the environment during all phases of the lifecycle of plastics. Different plastics can leach different additives at varying rates. If a polymer has a higher permeability, then it can leach additives at a faster rate.
Different physical structures of plastics such as hard crystalline or rubbery flexible plastic can affect the leaching process. More flexible or rubbery polymers have larger gaps in the polymer structure and can leach more
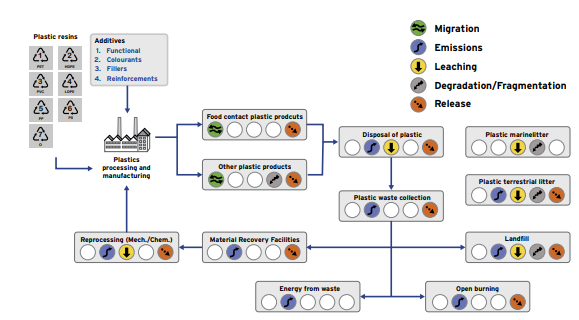
Figure 5. The pathways by which additives to common plastics reach the environment.
readily than a hard, crystalline structure. In addition, a low molecular weight additive can leach more quickly from the polymer matrix. The amount of additive that can leach from plastic depends on many factors, including weathering and temperature, but is ultimately limited by its proportion of the total plastic mass, which can be surprisingly high. PVC can contain more than 40% by weight of plasticizers such as phthalates .
Mechanical recycling cannot separate additives from the recyclate it creates. Chemical recycling has much greater ability to do so because it reduces plastic waste to molecular, monomer or polymer levels, separating them from the additives.
Of all technologies discussed below for management of plastic waste, supercritical and subcritical water oxidation (SCWO and iSCWO) are the only depolymerization technologies that have a recyclate output that does not contain the residues of chemical additives, a waste rich in toxic additive residue or emissions that convert additives to unintentionally produced POPs (UPOPs), (as is the case with incineration and pyrolysis technologies). The additives, including POPs, are destroyed in the depolymerization process. Depolymerization of plastics using SCWO and iSCWO have discharges of carbon dioxide, water, and depending on the waste feed, salts and/or metallic oxides. Steam is vented to the atmosphere. There are no particulates released or pollution-abatement filters required.
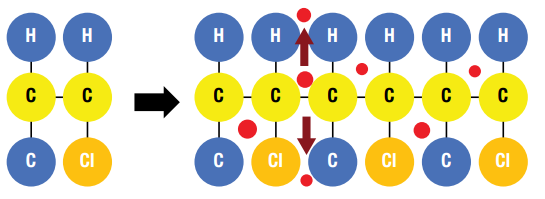
Figure 6. Additives (red dots) are not chemically bound to the polymer chain and can leach out of the plastic structure.
Clean water is produced requiring no pre-treatment before sewer disposal (elevated salinity and metal oxides limit the use of the effluent water).