WHAT ARE MONOMERS, POLYMERS AND PLASTICS?
Plastics are essentially large units of smaller linked molecular building blocks called ‘monomers’.
Monomers are made up of molecules which in turn are a group of atoms bonded together, representing the smallest fundamental unit of a chemical compound that can take part in a chemical reaction. When the monomers are joined together in chains and/or branching structures they are known as ‘polymers’. Linear polymers (a single linear chain of monomers) and branched polymers (linear with side chains) are thermoplastic; they soften when heated. Cross-linked polymers, that is polymers with bonds formed between polymer chains, either between different chains or between different parts of the same chain, are thermosetting and harden when heated .
Different types of polymers are made by linking together monomers with different chemical compositions using either linear or branched structures. These different types of polymers are what we commonly refer to as plastics, but each polymer also has a technical name based on its chemical and structural composition e.g. low density polyethylene, polyvinyl chloride and so on.
The chemical bonding process of linking monomers into polymers is called polymerization. Forming a single polymer may require thousands of monomers in chains. Common processes for polymerization include:
· Condensation polymerization – where the joining or polymerization is by water, carbon dioxide or ammonia. During condensation, two monomers join together and lose smaller molecules such as water or methanol. This process can be reversed during chemical recycling operations such as ‘depolymerization
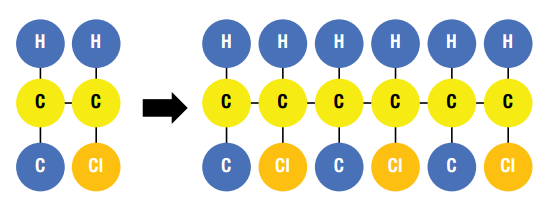
In this figure the monomer to the left is Vinyl Chloride. When linked together in chains the Vinyl Chloride molecules form Poly Vinyl Chloride or PVC.
· Addition polymerization (polyaddition) – where the join is formed by alkene catalysts such as ethene or propene.
Before the polymers can become a final plastic product that are treated with chemical additives that impart certain properties deemed desirable in the final product (UV-resistance, flexibility, color etc).
At this point the basic polymer material (pellets, nurdles or flakes) having been treated with additives can be cast, spun, extruded or otherwise shaped into the final plastic product.
These basic chemical and structural elements of plastics or polymers become important in the processes of mechanical and chemical recycling discussed below, as many of the challenges in recycling plastic relate to separating and reconstituting or mixing polymers.
Most currently produced plastics are made from petrochemical feedstock. Chemical engineering of petrochemicals allows for a vast array of different types of polymers, to be produced at industrial scale.
Global demand for plastic is dominated by a handful of polymers of the thermoplastic type: polypropylene (PP) (21%), low- and linear low-density polyethylene (LDPE and LLDPE) (18%), polyvinyl chloride (PVC) (17%), and high-density polyethylene (HDPE) (15%). Other plastic types with high demand are polystyrene (PS), and expandable PS (8%), polyethylene terephthalate (PET) (7%, excluding PET fibre) and the thermosetting plastic polyurethane .
The sectors that generate plastic waste are dominated by packaging, textiles, consumer products, transport, construction, and electronics (Figure 4). Clearly the packaging sector is by far the greatest generator of plastic waste (much of it is single-use packaging), which is why it has been in the spotlight for visible plastic pollution in the environment.