PLASTIC TO NON-FOSSIL FUELS (HYDROGEN)
Less attention has been paid to the conversion of plastic waste to hydrogen – a non-polluting, non-fossil fuel. A few industrial-scale pilot plants have been established to convert unrecyclable mixed plastic waste to hydrogen fuel. When hydrogen is used as a fuel in vehicles or stationary sources via a hydrogen fuel cell, the outputs are water and warm air. At face value the use of hydrogen fuel in a vehicle creates almost no carbon emissions compared to fossil fuels produced via refineries or derived from plastics. However, the method used to produce the hydrogen can significantly affect the overall global warming potential of the process. To denote the energy intensiveness of hydrogen production by different methods, the titles Green hydrogen, Blue hydrogen, and Grey hydrogen have been applied.
THE GLOBAL WARMING POTENTIAL OF FOSSIL FUEL DERIVED FROM PLASTIC IS VERY HIGH.
Green hydrogen is hydrogen produced via electrolysis using low-carbon renewable energy such as solar and wind power. Grey Hydrogen, accounting for around 98% of total hydrogen production today, is produced via steam methane reforming of natural gas in the petrochemical industry, without any attempt to offset carbon emissions, and represents a highcarbon pathway to producing hydrogen. Blue hydrogen involves the same
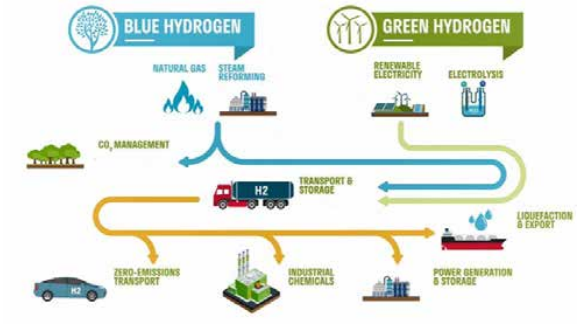
Figure 21. Green and blue hydrogen production pathways.
petrochemical process (Figure 21), and is also a relatively high-carbon pathway to produce hydrogen, but is claimed to be cleaner as it involves the offset, capture, and storage or reuse of the carbon emissions.
The hydrogen economy is seen as key transition to a low-carbon future.26 Vehicles, buildings (McClarty et al., 2016), and even steel mills27 can now be powered by hydrogen. However, this is largely dependent to the degree to which Green hydrogen can move from a minor to major supplier of hydrogen within the economy. Currently Grey and Blue hydrogen are much cheaper, but the price of Green hydrogen is expected to fall considerably over the next decade, while carbon pricing may force the price of Grey and Blue hydrogen higher.
Currently there are two pathways to generate hydrogen from plastic waste: pyrolysis and photoreformation.
Pyrolysis
Pyrolysis is used to convert the plastic waste to syngas, char, and tar. The syngas consists mostly of methane, carbon monoxide, and hydrogen. The hydrogen can then be isolated from the rest of the syngas components, which are combusted for energy. In essence, this is a different version of Grey hydrogen production.
This process suffers from linearity, emissions, and residues at the pyrolytic stage, and from a relatively high carbon footprint considering the embedded energy in creating the plastic, sorting and preparing it for pyrolysis, as well as the energy use in the pyrolysis plant to produce the hydrogen. However, it may represent a potentially sounder environmental outcome for unrecyclable plastic waste than incineration or dumping. It certainly represents a better outcome than pyrolysis producing fossil fuel. While the hydrogen may be a ‘clean fuel’ the methane and other constituents of the syngas (including contaminants such as dioxin) will still be combusted, posing similar problems as plastic to fossil fuel. Pilot plants are currently proposed to be established in the UK by PowerHouse Energy and Waste2tricity28. There is currently no industry established beyond these pilot proposals.
Photoreformation
Photoreformation is used to convert plastic waste to hydrogen. An emerging technology is being developed to generate hydrogen from unrecyclable plastic waste without the disadvantages of the pyrolysis process. Scientists in the UK have developed a system using cadmium sulfide quantum dots as photocatalysts to degrade plastics in the presence of sunlight and generate hydrogen. The process operates under ambient temperature and pressure, generates pure hydrogen, and converts the waste polymer into organic products such as formate, acetate, and pyruvate .
Annika Friberg of Chemistry World reports that,
“They drop the photocatalyst onto the plastic then immerse the plastic in an alkaline solution. Irradiation with sunlight reduces water from the solution to hydrogen while the plastic polymers simultaneously oxidize to small organic molecules. The group tested the system by photoreforming three common polymers; polylactic acid, polyethylene terephthalate and polyurethane. The results matched those of state-of-the-art hydrogen evolution photocatalysis systems that employed expensive sacrificial reagents.”
The process, as developed so far, operates efficiently irrespective of external organic contaminants on the plastic waste or embedded additives. While the technique is now in the process of a scale-up it has not yet been operated at pilot scale. While again it is essentially a linear process, it does have useful end-product chemicals, as well as hydrogen for energy. It could potentially be a simpler, less environmentally damaging way to manage large existing stockpiles of unrecyclable plastic waste, without the negative impacts of pyrolysis .
