CHEMICAL RECYCLING TECHNIQUES
The three main classes of chemical recycling techniques are:
1. Chemical depolymerization: a chemical-based process that converts plastic waste back into monomers using chemical reactions. It is suitable only for homogenous pre-sorted plastic waste streams such as PET, PU, PA, PLA, PC, PHA, and PEF.
2. Solvent-based regeneration: A purification process based on dissolving polymers in proprietary solvents, separating contaminants and reconstituting the target polymer. The process can accommodate a variety of plastics.
3. Thermal depolymerization and cracking (gasification and pyrolysis): These processes heat plastic waste in a low-oxygen environment to produce molecules from mixed streams of monomers that then form the basis of feedstock for new plastic without degradation. The main output is syngas or synthesis gas.22 Both gasification and pyrolysis have been trialed for decades to create energy (syngas burned to drive steam turbines) from municipal waste, but have not been a commercial success due to a combination of poor economics, high energy consumption requiring supplemental fuel, fires, explosions, emissions, and residues. These processes are also used to create ‘plastic to fuels’ (fossil fuels), as oils and diesel can be generated in addition to syngas. Figure 15 provides a flow chart showing the spectrum of mechanical, chemical, and solvent-based recycling applications.
Chemical depolymerization
This chemical recycling process is essentially the opposite of polymerization, described in figure 3, and produces single monomer molecules or shorter fragments called oligomers. The process only operates efficiently with highly selective inputs requiring careful source segregation and is well suited to PET and purified terephthalic acid (PTA) but is also applicable to PA, PU, PLA, PHA, PEF, and PC and a range of polyesters.
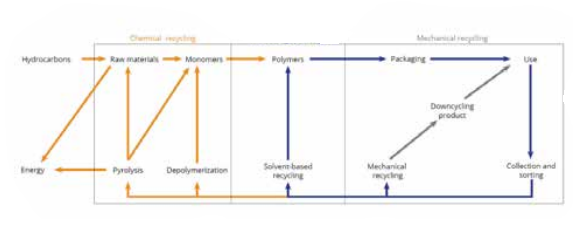
Figure 15. Spectrum of chemical recycling through solvent to mechanical recycling.
Depolymerization produces monomers which must again be polymerized (additives must also be incorporated to replace those lost) to produce plastics, whereas solvent-based regeneration processes produce a purified polymer ready for conversion to plastic products. In both depolymerization and solvent regeneration, colorants, additives, and other contaminants can be completely separated at the molecular level (if processes are followed to strict standards), and the resulting output is of high purity . This level of purity is difficult to achieve with mechanical recycling unless a very clean input is used. Chemical depolymerization does not currently operate on a large-scale commercial basis, only industrial pilot plant and lab-scale operations exist. There has only been one large commercial-scale PVC solvent recycling plant (VinyLoop plant in Italy, a joint venture 10 000 tons/year facility,) operated since 2002, but it was shut down in 2018 because it could not economically separate the substantial amounts of phthalate plasticizers used in soft PVC to meet EU regulatory requirements .
AS WITH MOST CHEMICAL RECYCLING TECHNOLOGY THE TOXICITY, FATE, AND CHARACTERISTICS OF THE RESIDUES CREATED BY DECONTAMINATING THE MONOMERS HAS NOT BEEN MADE PUBLIC. THE HAZARDS ASSOCIATED WITH THE PROPRIETARY CATALYSTS USED IN DEPOLYMERIZATION HAVE NOT BEEN DISCLOSED.
Depolymerization allows the output monomers to be used separately or mixed to create virgin-grade polymers. PET is an example where the monomer output can be used flexibly and either applied to packaging or textiles (PET fibres in textiles are known as polyester). This could be a positive development, as most textile polymers are currently not recycled, yet make up 60% of the production use of PET, while the remaining volume is used mostly for packaging .
Given that PET accounts for 18% of global plastic production , recycling PET textiles (less than 1% are currently recycled) and marine PET litter via depolymerization could potentially reduce this type of plastic pollution. Marine PET litter may have the added complication that it adsorbs and concentrates toxic POPs from ocean waters. Adsorption is the adhesion of molecules from a gas, liquid or dissolved solid to a surface. In this case to the surface of the PET plastic. If contaminated PET is then subject to depolymerisation the POP contaminants would be separated and form part of the toxic residue of the process generating more hazardous waste for disposal.
Energy use in depolymerization to cleave molecular chains and recover monomers depends on the target polymer. Currently energy use and other costs in this type of recycled polymer production are significantly higher than in virgin polymer production. However, the Global Warming Potential (GWP), a measurement of the carbon footprint of production, for plastics created via depolymerization is only around 60% of that of virgin plastics (Crippa et al., 2019), which in time, may level the field in terms of cost structures – particularly if carbon pricing is implemented more widely. As with most chemical recycling technology the toxicity, fate, and characteristics of the residues created by decontaminating the monomers has not been made public. The hazards associated with the proprietary catalysts used in depolymerization have not been disclosed.