PHASE EQUILIBRIA IN POLYMER SYSTEMS
Many polymer products are multi-component mixtures of polymers or of polymers and other compounds such as solvents, monomers and modifiers like plasticizers, tackifiers etc. Understanding the phase behavior of these mixtures is essential to obtain the desired blend properties.
In most cases, mixturers of polymers are not miscible, that is, only very few pairs of polymers are known to be miscible and then only in a narrow temperature and concentration range. The main factors that determine the phase behavior are
- Average molecular weight and distribution of molecular weights
- Structure and chemical composition of the polymers and additives/modifiers
- Polymer concentration or polymer volume fraction in the blend
- Temperature and pressure
Over the last seventy five years, a large number of models have been developed to describe the phase behavior of polymer blends and of polymers in solvents. A comprehensive description of these models can be found in the book "Thermodynamics of Polymer Blends" by Yuri Lipatov and Anatoly Nesterov.1 The models can be divided into four broad classes
- Lattice models based on the Flory-Huggins theory such as the compressible regular2 solution free energy model (CRS)
- Equation of state (EOS) models for lattice fluids such as Flory-Orwell-Vrij and Prigogine-Sanchez-Lacombe model
- Free-volume activity coefficient models, based on UNIQUAC/UNIFAC group contribution approach
- Models based on perturbation theory, such as the perturbed-chain statistical associating fluid theory (PC-SAFT)
The thermodynamic driving force for mixing is the the osmotic pressure Π. This quantity is always positive. However, for large molecules, Π is relative small. This follows dircetly from the osmotic pressure relationship:
Π ≈ RT c Mn-1
where c is the polymer concentration which is equal to the polymer volume fraction divided by its specific volume, c = φp / vp and Mn the number average molecular weight.
The osmotic pressure rapidly decreases with increasing molecular weight, Mn(molecule size), and is very small for high molecular weights. Since mixing of polymers is an endothermic process, at least for the majority of polymer blends, most pairs of polymers are not miscible; or in other words, blends of two or more polymers are only miscible if special exothermal interactions between the repeat units of the two diffferent polymers are present, like hydrogen bonding, or when the polymers have a similar chemical structure (solubility parameters).
From the thermodynamic models of polymer solutions and polymer blends the conditions (temperature, pressure, composition) under which phase separation occurs can be predicted. The phase diagrams for two components can resemble any of those shown below.
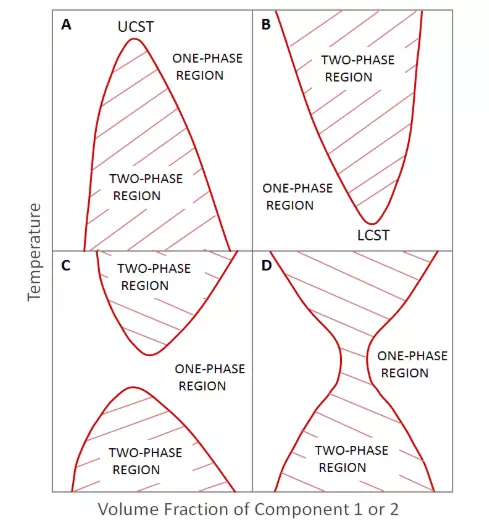
The clasical Flory-Huggins theory of mixing predicts only one region of immiscibility with an upper critical solution temperature. However, for some polymer blends, there are two regions of immiscibility; one is characterized by an upper critical solution temperature (UCST) and the other by a lower critical solution temperature (LCST). A third polymer blend system shows one continuous region of immiscibility extending from low to high temperatures with miscibilty at (very) low and high polymer volume fractions.