OSMOTIC PRESSURE OF POLYMER SYSTEMS
The osmotic pressure is the minimum pressure that has to be applied to a polymer solution to prevent a solvent to flow through a semipermeable membrane that separates pure solvent from polymer dissolved in the same solvent.
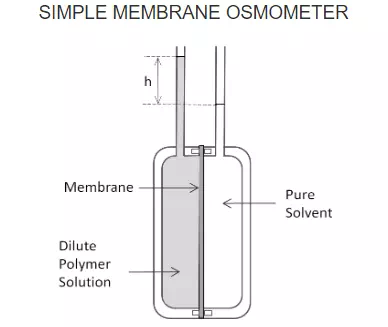
Osmometry is frequently used to determine the number-average molecular weight of polymers. A basic osmometer design is shown in the Figure below.
The osmotic pressure is also the thermodynamic driving force for mixing. It is always positive. However, for large molecules, this force is relative small. This follows directly from the osmotic pressure relationship:
Π = RT ln [as] / Vs
where Vs is the molar volume of the solvent and as is its activity. For polymer-solvent blends, the solvent activity can be calculated with the Flory-Huggins expression for activities:1,2
ln [as] = Δμs/kT ≈ ln [1- φp] + φp + χpsφp2
where φp is the polymer volume fraction. Substitution of the Flory-Huggins expression for solvent activity into the osmotic pressure relation gives
Π ≈ RT Vs-1 · {ln [1- φp] + φp + χpsφp2}
This expression can be simplified by expanding the logarithmic term in a Taylor series:
Π ≈ RT Vs-1 · {φp / N + φp2 · (0.5 - χ) + φp3 +...}
or
Π / c ≈ RT Mn-1 · {1 + (Mnvp2/Vs) · (0.5 - χ) c + (Mnvp3/Vs) c2/3 + ...}
where vp is the specific volume of the polymer, c the polymer concetration, c = φp / vp, and χ the Flory-Huggins interaction parameter. The equation above can be rearranged to give the widely used virial expansion relation for the osmotic pressure3
Π / c ≈ RT · {Mn-1 + A2 c + A3 c2 + ...}
where A2 and A3 are the second and third virial coefficients:
A2 = vp2/Vs · (0.5 - χ0)
A3 = (vp3/Vs) / 3
Values of the the second (A2) and third osmotic virial coefficient (A3), defined by the equation above, have been determined for many polymers, for example by light-scattering or osmotic pressure measurements. They are often tabulated as a function of polymer moelcular weight. Of great importance is the second osmotic virial coefficnent A2, because the polymer-solvent interaction parameter χ0 (at infinit dilution) can be calculated from it using the equation:
χ0 = 0.5 - Vs A2 / vp2 = 0.5 - Vs A2 ρp2
For very dilute ideal polymer solutions, χ ≈ 0.5, φ → 0, the second and third term can be neglected. This gives the classical van't Hoff equation for the osmotic pressure of an ideal, dilute solution:
Π / c ≈ RT Mn-1
According to this equation, the osmotic pressure of an ideal polymer solution rapidly decreases with increasing molecular weight, i.e., the driving force for polymer-polymer mixing is rather small.
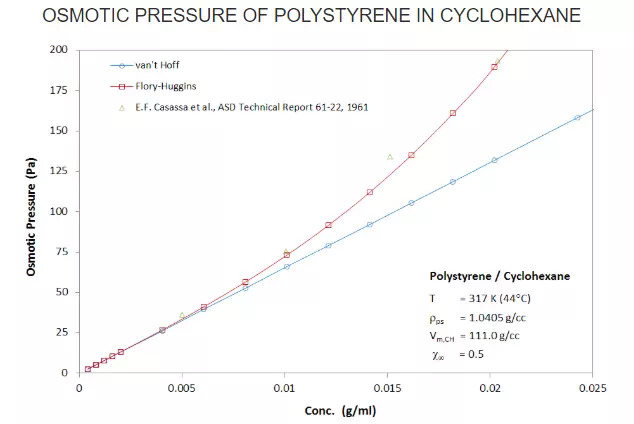
In the Figure below, plots of the osmotic pressure for different concentrations of polystyrene (Mn = 404000 g/mol) in cyclohexane are shown. For low polymer concentrations in a good solvent (χ = 0.5), the osmotic pressure can be calculated with the vant Hoff law. However, for concentrations above 0.1 g/cc, the fit is poor and Flory's virial expansion relation gives much better results.