HANSEN SOLUBILITY PARAMETERS
Hansen solubility parameter were developed by Charles M Hansen in 1967 to guide solvent selection in the paint and coatings industry. Today, they are widely used in many other fields. The basis of these so-called Hansen solubility parameters (HSP) is that the total cohesive energy can be separated in three parts. These arise from (atomic) dispersion forces, (molecular) permanent dipole–permanent dipole forces, and hydrogen bonding:
Et = Ed + Ep + Eh
δt2 = δd2 + δp2 + δh2
The total cohesive energy of monomers and solvents, Et, can be calculated from the energy of vaporization, Et ≈ ΔHvap - RT, whereas the cohesive energy of polymers has to be indirectly measured, for example, by measuring the cloud point (dissolved polymers) or by measuring the maximum of swelling (crosslinked polymers) with a series of solvents or solvent mixtures with known solubility parameters.
The partial energies and solubility parameters can only be calculated. For example, they can be predicted with equation-of-state methods (EOS) or by establishing correlations between the Hansen solubility parameters (energies) and other physical properties of the substance. The later approach had been chosen by Koehnen and Smolders (1975) who found correlations between the partial solubility parameters and other thermo-physical properties like surface tension, dipole moment, and index of refraction. Many other correlations have been found over the years. Some of these relations will be presented below.
Koehnen and Smolders were one of the first to search for relationships between the cohesive energy density (CED) and the index of refraction. They found for nonpolar solvents following relationship:1
δd = 19.53 nD - 11.35
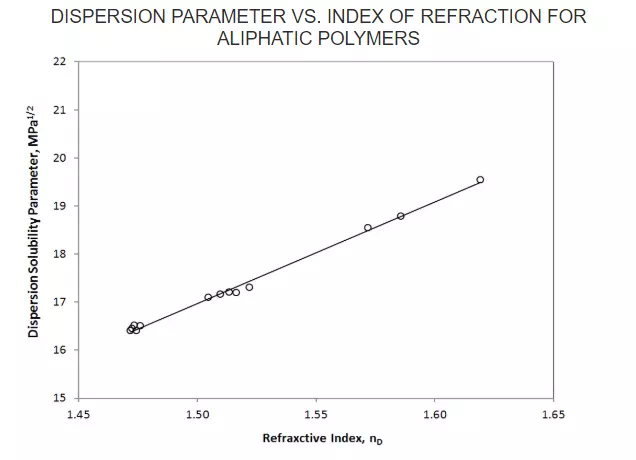
A similar relationship can be found for aliphatic polymers (see Fig. below):
δd = 20.80 nD - 14.21
Koehnen and Smolders postulated that the relationships above are also valid for polar substances, at least in first approximation. However, Hansen2 suggested that a procedure developed by Blank and Prausnitz should be used to estimate the dispersion parameter, δd. This method is based on the assumption that the dispersion component of the cohesive energy of the molecule in question is equal to that of a hypothetical nonpolar molecule of same volume and structure but without any functional groups, i.e. to a hydrocarbon molecule of same volume and structure.
The contribution of the permanent dipoles to the cohesive energy density (solubility parameter) can be predicted with a formula proposed by Boettcher3
δp2 = 12100 (ε - 1) μ2 (nD2 + 2) / [Vm2(2ε + nD2)]
where ε is the dielectric constant and μ is the dipole moment. If these parameters are not available, which is the case for many substances, Hansen and Berrbower suggest following simpler relationship:4
δp = 37.4 μ / Vm1/2
Hansen and Beeerbower have tested this formula for many compounds and found it to be reliable. If the dipole moment is unknown or unreliable due to the small dipole moment of the compound in question, the polar parameter should be predicted with group contribution methods.
The third parameter, the so-called hydrogen bond parameter, δh, is usually found by subtracting the polar and dispersion parameters from the total parameter, or in terms of energies, by subtracting the dispersion and polar energies from the cohesive energy. If the total cohesive parameter (energy) is unknown, several methods are available to calculate it.The hydrogen bond energy can also be predicted directly with group contribution methods (see table below). However, this approach is less accurate and not recommended.
ESTIMATED HYDROGEN BONDING ENTHALPIES |
|
|
Functional Group |
Enthalpy of an H-bond |
|
Ester Group, -COO- |
1250 ± 150 cal/mole |
|
Acid Group, -COOH |
2750 ± 250 cal/mole |
|
Ketone Group, >CO |
800 ± 250 cal/mole |
|
Ether Group, -O- |
450 ± 25 cal/mole |
|
Phenylene Ring |
50 ± 50 cal/mole |
|
Nitrile Group, -CN |
500 ± 200 cal/mole |