High-Pressure Polymerization
High pressure polymerization is a free radical process using oxygen, peroxides or other strong oxidizing chemicals to initiate the reaction. It produces low density polyethlyene (LDPE) with a density range of 0.919 to 0.940 g/cm3. High pressure itself refers to 60 to 350 MPa.
In this process, ethylene is polymerized to polyethylene using very high pressures and free-radical catalysts. Depending on the specific product to be manufactured, different modifiers can be fed to the process along with the ethylene forming highly branched polymers.
Because of safety and cost-efficiency issues, high-pressure polymerization has declined in its use in favor of low-pressue polymerization.
Low-Pressure Polymerization
Low-Pressure polymerization uses heterogeneous catalyst supported on an inorganic carrier, or Ziegler catalysit such as aluminum alkyls and titanium halides. It produces high density polyethylene (HDPE) with a density range of 0.941 to 0.970 g/cm3.
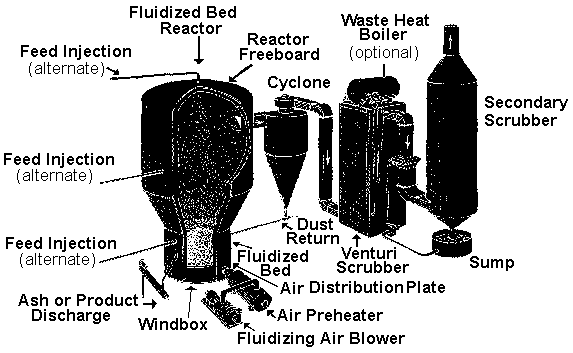
The reactors run at pressures ranging from 0.1 to 20 MPa and temperatures of around 50 to 300oC.
Because the extrem conditions of the high-pressure process are not present, low-pressure dominates current polyethelene manufacturing industry.