Conducting polymers
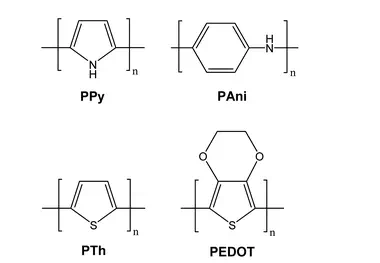
The derivatives of polypyrrole with resistivities as low as 1 Ω cm were first reported in 1963 by Australian scientists Bolto and Weiss, and their coworkers. The discovery of polyacetylene and its high conductivity upon doping in by Shirakawa and coworkers in the 1970s further helped to advance the field of conductive polymers. Polypyrrole (PPy), polyaniline (PAni), polythiophene (PTh), and poly(3,4-ethylenedioxythiophene) (PEDOT) are most promising conducting polymers (CPs) for use in biomedical applications. CPs exhibit electrical and optical properties similar to those of metals and semiconductors, and offer advantages of conventional polymers such as ease of synthesis. As an electrode for stimulation and recording, conducting polymers are attractive due to the possibility of chemical surface modification with physiologically active species to enhance the biocompatibility and the functionality of the electrodes. This unique combination of properties makes CPs potential candidates for various biomedical applications such as biosensors, neural probes, neural prostheses, drug-delivery devices, tissue-engineering scaffolds, and bio-actuators.
The presence of conjugated double bonds (Fig. 2) along the backbone gives rise to the conductivity in CPs. The π electrons in the conjugated backbone are available to delocalize into a conduction band and in the idealized situation of a uniform chain, the resulting conduction band would give rise to metallic behaviour. However, such a system is unstable with respect to bond alternation, which causes the formation of an energy gap in the electronic spectrum. Dopant ions are introduced to the structure to overcome the energy gap and hence, to impart conductivity to these polymers. The dopant ions carry charge in the form of extra electrons to neutralise the unstable backbone of the polymer in its oxidised state by donating or accepting electrons.18,19 On application of a potential across the CP film, a charge is passed through the film as a result of a flux of ions either in or out of the film, dependent on dopant charge and mobility, causing a disruption to the polymer backbone. CPs can be doped with both p- and n-type dopants using a variety of molecules, such as small salt ions (Cl−, Br−, or NO3−), and larger dopants such as hyaluronic acid, peptides or polymers.
|
Fig. 2 Structures of common CPs investigated for biomedical applications. |
||
CPs can be synthesized either chemically or electrochemically. Chemical methods of CP synthesis either use condensation polymerization or addition polymerization. While chemical synthesis provides many different possible routes to synthesize a variety of CPs and also permits the scale-up of these materials, electrochemical synthesis is relatively straightforward and hence, is most commonly used for making CPs. The advantages of electrochemical synthesis include ease of synthesis, simultaneous doping and entrapment of molecules during synthesis, however the films are difficult to remove from electrodes and post-synthesis covalent modification of CP is difficult. On the other hand, chemical synthesis offers more options to modify CP backbone covalently and makes the post-synthesis covalent modification possible, although this method is often more complicated. Another significant difference between electrochemical and chemical methods of CP synthesis is that electrochemical method can produce very thin CP films (of the order of 20 nm), whereas powders or very thick films are usually produced with chemical polymerization. Furthermore, electrochemical synthesis is limited to those systems in which the monomer can be oxidized upon application of potential to form reactive radical ion intermediates for polymerization. The common CPs (e.g. PPy, PTh, PAni, PEDOT) can be polymerized both chemically and electrochemically; however, several novel CPs with modified monomers can only be synthesised using chemical polymerization.
Table 1 presents the properties and electrical conductivity of some conventional conducting polymers investigated for biomedical applications.
Table 1 Conductivity and other properties of common conjugated conducting polymers used for biomedical applications
|
Polymer |
Conductivity (S cm−1) |
Type of doping |
Properties |
Limitations |
|
|
Polypyrrole |
10–7.5 × 103 |
p |
High electrical conductivity, ease of preparation and ease of surface modification |
Rigid, brittle and insoluble |
|
|
Polyaniline |
30–200 |
n, p |
Diverse structural forms, environmentally stable, low cost |
Hard to process, non-biodegradable, limited solubility |
|
|
Polythiophene |
10–103 |
P |
High electrical conductivity, ease of preparation, good optical property |
Hard to process |
|
|
Poly(3,4-ethylene dioxythiophene) |
0.4–400 |
n, p |
Transparent conductor, environmentally and electrochemically stable |
Limited solubility |
|