FLORY-HUGGINS LATTICE THEORY OF POLYMER SOLUTIONS, PART 1
The thermodynamics of (binary) regular polymer solutions1 were first investigated by Paul Flory2 and Maurice Huggins3 independently in the early 1940s. They assumed a rigid lattice frame, that is, the molecules in the pure liquids and in their solution / mixture are considered to be distributed over N0 lattice sites, as illustrated in the figure below. The total number of lattice sites, N0, is assumed to be equal to the number of solvent molecules, Ns, and polymer repeat units, Npr:
N0 = Ns + Npr
where Np is the number of polymer molecules each consisting of r repeat units.
TWO-DIMENSIONAL LATTICE
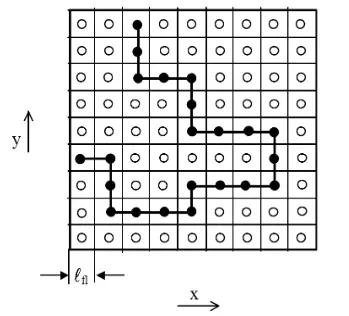
The model has been described in great detail by Flory in his famous book "Principles of Polymer Chemistry" (1953).4 Following the standard theory of mixing for small molecules of similar size and using Stirling's approximation lnM! = M lnM - M, Flory and Huggins obtained following expression for the entropy of mixing:
ΔSmix ≈ -k (Np ln φp + Ns ln φs )
Alternately, the entropy of mixing can be written as
Δsmix = ΔSmix / N0 ≈ -k {φp / (r·vr) · ln φp + φs / vs · ln φs}
where φs and φp are the volume fractions of the solvent and polymer,
φs = Nsvs / (Nsvs + Nprvr), φp = Nprvr / (Nsvs + Nprvr)
and vs and vr are the volumes of a solvent molecule and of a polymer repeat unit, respectively. Obviously, the solvent needs not necessarily to be made of a single unit. The solvent may, in fact, consist of several repeat units or of another polymer.
The Gibbs free energy of mixing, ΔGmix, often includes an enthalpy part, that is, mixing can be an endothermic or an exothermic process
ΔGmix = ΔHmix - T ΔSmix
where ΔHmix is the heat of mixing. Flory and Huggins introduced a new parameter, the so called Flory-Huggins interaction parameter to describe the the polymer-solvent interaction:5
χps = ΔHmix / (kT Ns φp)
which combined with the entropy term leads to the free energy of mixing:
ΔGmix / kT = Np ln φp + Ns ln φs + χps Ns φp
Assuming equal lattice volumes for both repeat units, the free energy per lattice site can be written
Δgmix / kT ≈ φp / r · ln φp + φs ln φs + χps φs φp
A more general expression for the free energy of mixing is
Δg'mix / kT = φp / (r·vr) · ln φp + φs / vs · ln φs + χps φs φp / √(vrvs)
where Δg'mix is the free energy of mixing per unit volume. These equations are the starting point for many other important equations. For example, the partial molar free energy of mixing (chemical potential) can be obtained by differentiation of the expression above with respect to Ns. This gives
Δμs/RT = ln [1- φp] + (1 - 1/r) φp + χps φp2
where μs is the chemical potential of the solvent per mole. Substitution of this expression into the osmotic pressure relation Π = - Δμs / Vs gives
Π ≈ RT Vs-1 · {ln [1- φp] + φp + χpsφp2}
where Vs is the molar volume of the solvent.