POLYMER PHASE EQUILIBRIA
SPINODAL AND BINODAL
From the thermodynamic models for polymer blends and dissolved polymers, it is possible to calculate the thermodynamic conditions (temperature, pressure, and composition) under which phase separation occurs.
Whether two polymers are mutually miscible or a polymer is soluble in a solvent, depends on the sign of the Gibbs free energy, Δgm, which is related to the energy and entropy of mixing:
Δgmix = Δhmix - T Δsmix = Δhmix - T ∂Δgmix / ∂T |p,φ < 0
However, this criterion alone is not sufficient to determine if a mixture is truly stable against phasing into two polymer mixtures of different composition. It only states that the mixture will not separate into two phases of pure components, like pure solvent and polymer. As will be shown below, phasing can occur even for negative Gibbs free energies of mixing, Δgmix < 0, whereas a mixture is stable if the free energy curve does not show local minima.
Δgmix < 0 and ∂2Δgmix / ∂φp2
The dependency of Δgmix on composition (i.e. volume fraction) at constant temperature is illustrated in the figure below. If Δgmix is positive over the entire range of composition, as illustrated by curve I, then the two components are completely immiscible, or in other words, the solvent and the polymer form two separate phases. The other extreme, is total miscibiltiy, which is illustrated by curve III. This is the case when Δgmix is positive and the second derivative of Δgmix is negative over the entire range of composition:
Δgmix < 0 and (∂2Δgmix / ∂φP2)p,T > 0
GIBBS FREE ENERGY OF MIXING AS A FUNCTION OF COMPOSITION
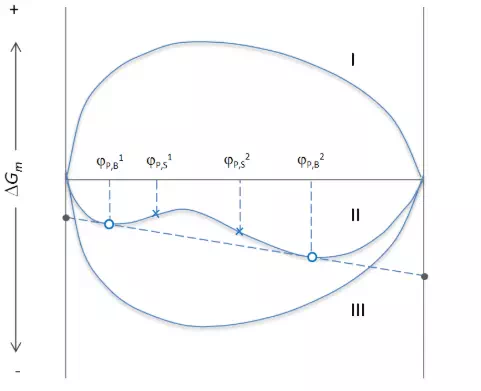
The third case describes partial miscibility. This curve has two minima at φP,B1 and φP,B2. Any mixture with a composition between these two concentrations will spontaneously phase separate into a solvent-rich and polymer-rich phase of composition φP,B1 and φP,B2 which are the points of the common tangent as illustrated by curve II. For a given polymer fraction φP, the volume fractions φP,L and φP,H of the two coexisting mixed phases can be calculated from
φP = φP,L φP,B1 + φP,H φP,B2
= φP,L φP,B1 +
(1 - φP,L) φP,B2
Solving this equation for φP,l gives
φP,L = (φP,B2 - φP) / (φP,B2 - φP,B1)
φP,H = (φP - φP,B1) / (φP,B2 - φP,B1)
The two minima φP,B1 and φP,B2 are the so called binodal points, which can be found by differentiating the Gibbs free energy of mixing, Δgmix, with respect to the composition, φP. At constant pressure (p) and temperature (T), the binodal condition reads
∂Δgmix / ∂φP |φP,B1 = ∂Δgmix / ∂φP |φP,B2-
the condition for equilibrium between the two phases in a binary mixture can be also expressed by equality of the chemical potentials in the two phases:
μP11 = μP12, and μP21 = μP22
μPi = ∂Δgmix / ∂ni |p,T, nj
where the subscripts P1 and P2 are the designations for the two compounds and the superscripts 1 and 2 for the two phases.
For a symmetrical polymer blend, i.e. for a blend of two polymers with equal chain length, rP1 = rP2 = r, the expression for the binodal reads:
χ1,2 · r = ln[φP2 / φP1 ] / (1 - 2φP1)
where φP1 and φP2 are the volume fractions of the two polymers and χ1,2 is the polymer-polymer interaction parameter.
For the general case rP1 ≠ rP2, no simple analytical solution exists, thus, the binodal points are usually estimated using numerical methods. This is often done with the aid of a computer. The binodal points can be also predicted by constructing the common tangent, as indicated in the figure above.
A very different situation is encountered when the composition lies between φP,B1and φP,S1 or between φP,B2 and φP,S2 (see curve II), where φP,S1 and φP,S2 are the points of inflection. We already stated that points on a concave curve, (∂2Δgmix /∂φP2)p,T > 0, are stable against phase separation, simply because on a concave curve, the total free energy of neighboring compositions is greater than that of the mixture in question, i.e., the free energy will always increase when the mixture separates into any two phases. Similarly, a mixture on a concave part of a curve with a convex portions is stable against phase separation into compositions on the concave part, that is, all points that lie between the minimum and the point of inflection of the concave portion are stable in regard to neighboring compositions. However, these compositions are not stable against separation into phases of composition φP,B1 and φP,B2. Thus, the mixture is only stable against small concentration fluctuations, but not stable against large fluctuations. This is why this region is called meta-stable. The point that separates the unstable region from the meta-stable is called a spinodal point. It is the point of inflection at constant T and p:
(∂2Δgmix / ∂φP2)p,T = 0
Another important point is the point at which the two coexisting phases coincide. This point is called the critical point. In a binary phase diagram, these are the lower (LCST) and upper critical solution temperature (UCST) of the phase curves. It is also the point where the binodal and spinodal coincide. These points are easy to calculate; if the temperature increases, the local maxima diminish and eventually vanish, thus the points of inflection and the contact points on the double tangent approach each other until they coincide. At this particular point, both the second and the third derivative of Δgmix vanish:
(∂2Δgmix / ∂φP2)p,T = (∂3Δgmix / ∂φP3)p,T = 0
The critical value of χ where the miscibility gap begins is given by1,2
χc = ½ · (rP1-½ + rP2-½)2
And the critical concentration is located at
φP1,c = rP1½ / (rP1½ + rP2½)