POLYMER PHASE SEPARATION
NUCLEATION & GROWTH AND SPINODAL DECOMPOSITION
Polymer blends can either form homgenous mixtures or they can undergo phase separation. The formation of a two-phase structure is usually induced by a change in temperature or molecular weight.
Whether two polymers are mutually miscible or whether a polymer is soluble in a solvent depends on the composition and the shape of the free energy curve. In general, blends are miscible if the free energy of mixing is negative, Δgmix < 0. However, this criterion alone is not sufficient to determine if a mixture is truly stable against phasing into two polymer mixtures of different composition. It only states that the mixture will not separate into two phases of pure components, like pure solvent and polymer. To better understand the phase behavior of polymer blends and solutions, we have to understand how the shape of the free energy curve of mixing effects the stability of the system. The figure below shows as an example the Gibbs free energy per unit volume of a blend of 100k/100k polystyrene (PS) and poly(cyclohexyl methacrylate) (PCHMA) as a function of composition for four different temperatures.
GIBBS ENERGY OF MIXING AS A FUNCTION OF COMPOSITION
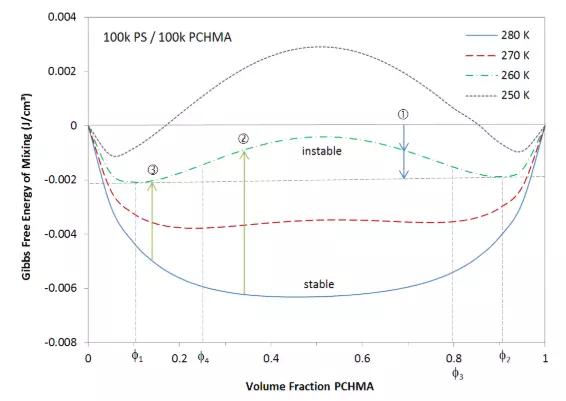
For high temperatures (T = 280K), the free energy of mixing shows only a minmum. In this case, the two polymers are miscible for all compositions, simply because any two-phases will have a higher free energy than any one-phase system. A change in phase behavior is observed when the curve displays a local maxima. This is the case, for the free energy curve at 260 K. Let us choose an arbitary composition, for example φPCHMA = 0.7. Two vertical arrows are drawn; the first arrow indicates that mixing of PS and PCHMA leads to a decrease in the Gibbs free energy when compared to two the free energy of pure PS and PCHMA. The second arrow shows that the free energy can be further decreased when the blend decomposes into two new blends of composition φPS ≈ 0.1 and φPS ≈ 0.9 which are the local minima. However, this kind of spontaneous decomposition is only possible if the composition lies between the two local minima:
φ1 ≤ φPCHMA ≤ φ2
Outside this range, phase separation into two new phases is not possible because a blend with lower polymer fraction cannot decompose into two phases of higher volume fraction.
Two different modes of phase separation can occur. The first mode is the so-called spinodal decomposition, which is equivalent with spantanous phasing. It occurs at concentrations between the two points of inflection, φ3 ≈ 0.25 and φ4 ≈ 0.8 (T = 260 K), because any random local fluctuation of composition will lead to a lower free energy, and thus, to spontaneous phasing. The second mode is nucleation and growth. This mode of phase separation occurs only in the regions φ1 < φPCHMA < φ4and φ2 > φPCHMA > φ3, which are the regions between the minima and adjacent points of inflection. Any composition in this region is meta-stable, because a small (thermally induced) fluctuation of concentration increases the free energy of the mixture. Only for larger fluctuations will the (local) mixture decompose into two new phases, i.e. the system has to overcome an activation barrier before it undergoes phasing.3 This type of phase separation leads to the formation of small spherical precipitates.