Ionic polymerisation
|
Alkenes can polymerise under the influence of both cationic and anionic initiators. As with free radical polymerisations, the ionic processes involve initiation, propagation and (sometimes) termination steps. Cationic Polymerisation
|

In ionic polymerisation there is no equivalent of termination by combination or disproportionation (as seen in free radical systems), but in cationic polymerisations polymer chains can terminate by proton transfer to monomer. However, this is clearly a chain-transfer process rather than a simple termination, as the resulting carbocation can initiate growth of a new polymer chain.

Anionic polymerisation
Anionic
polymerisations are typically initiated by carbanions such as organolithium
compounds.
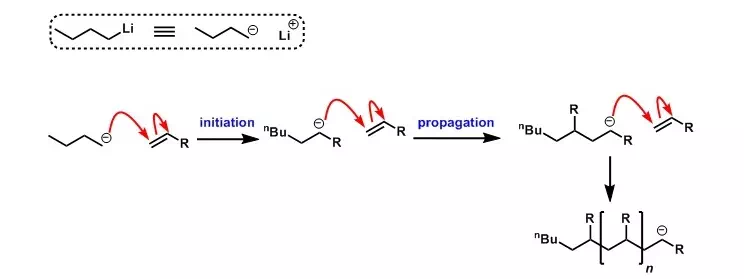
If carried out in a dry, aprotic solvent, anionic polymerisation has no significant termination or chain transfer processes, and can therefore be a "living" polymerisation. When the reaction ceases due to all monomer having been consumed, addition of further alkene (which can be a different alkene) results in further growth of the polymer chains.
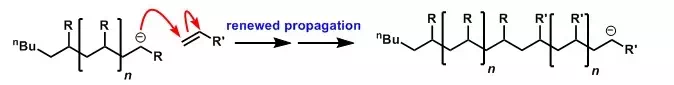
Finally, the polymerisation can be 'killed" by addition of a proton source such as an alcohol, which protonates the carbanionic centre.