SPECIALTY POLYMERS
Specialty polymers are polymers having properties and characteristics specially engineered for specific purposes. They are ranged from mono-functional to multifunctional polymers. Specialty polymers are specifically designed to serve a specific purpose.
They are made by keeping in mind where they are to be used, which chemical and mechanical properties are to be needed and how they to be shaped are. Every intricate detail is taken into consideration and their development is very sensitive if we talk about high end specialty polymers. They can be as cheap as commodity polymers to extremely expensive ones depending upon their area of application. Main classes of polymers which fall under the category of specialty polymers are as follows:
Conducting Polymers, Liquid Crystal Polymers, Biodegradable Polymers, Biomedical Polymers, Polymer Composites, Electroluminescent Polymers and more
LIQUID CRYSTAL POLYMERS (VECTRAN)
INTRODUCTION
A polymer that under suitable conditions of temperature, pressure & concentration, exist as liquid crystal is known as liquid crystal polymer and polymers that form liquid crystal stage contain long, rigid units or disc shaped molecular structure called as mesogens.
Liquid crystal polymers are sold by manufacturers under variety of trademarks like Kevlar,Vectran and Zenite 5145L. Liquid-crystal polymers (LCPs) are a class of aromatic polyester polymers. LCP (Liquid Crystal Polymers) are of two types based on their preparation process; lyotropic Liquid Crystal Polymers and Thermotropic Liquid Crystal Polymers.
In solid form, example of lyotropic LCP is Kevlar and example of Thermotropic LCP is Vectran.
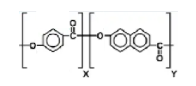
Molecular Structure of LCP Vectran
PROPERTIES
· LCP’s are extremely unreactive and inert, and highly resistant to fire.
· Environments that deteriorate the polymers are high-temperature steam,concentrated sulfuric acid, and boiling caustic materials.
· LCPs have a high mechanical strength at high temperatures, extreme chemicalresistance, inherent flame retardancy, and good weatherability
APPLICATIONS
· LCPs are useful for electrical and mechanical parts, food containers, and any otherapplications requiring chemical inertness and high strength.
· LCP is particularly attractive for microwave frequency electronics due to low relativedielectric constants, low dissipation factors, and commercial availability of laminates.
· Packaging Micro-electromechanical systems (MEMS) is another area that LCP hasrecently gained more attention.
· They are incorporated into various objects to enhance Fire retardant properties
ELECTROLUMINESCENT POLYMERS (POLYPHENYLENE-VINYLENE (PPV))
INTRODUCTION
Electroluminescence is a phenomenon in which a material emits light in response to the passage of an electric current or strong electric field and these are the polymers which emit light in response to the passage of an electric current or strong electric field.
Polyphenylene-vinylene (PPV) is the most common example of electroluminescent polymers. PPV (Polyphenylene-vinylene) exhibit electroluminescence. To generate light with these materials, a thin film of semiconducting polymers is sandwiched between two electrodes

Molecular Structure of PPV
PROPERTIES
· It is diamagnetic in nature & has a very low thermal conductivity (10–13 S/ cm).
· Tg is 85ºC and Tm is 285ºC.
· It is water soluble, but its precursors can be manipulated in aqueous solution.
APPLICATIONS
· It is capable of electroluminescence. Due to its stability, processability & electrical aswell as optical properties, PPV is used in organic light emitting diode (OLED).
· Devices based on PPV an emissive layer, emit bright yellow-green fluorescent light& derivatives of PPV are used when light of different color is required.
· It is also used as electron donating material in organic solar cells.
GLASS FIBRE REINFORCED POLYMER (GLASS FRP)
INTRODUCTION
Glass fibers are obtained by forcing glass melt through spinnerets (having small holes)and rapidly pulling & cooling to get fibers. Glass fiber reinforced polymer is a polymermatrix containing nylon, polyesters, etc
PROPERTIES
· Lower densities.
· Excellent resistance to corrosion & chemicals.
· They show applications in limited temperature range as Polymer matrix flows at hightemperature.
· High tensile strength & impact resistance
APPLICATIONS
· They are used in automobile parts, storage tanks, industrial flooring, plastic pipes,etc.
· They are extensively used in automobiles to reduce vehicle weight & boost fuelefficiency.
CARBON FIBRE REINFORCED POLYMER (CARBON FRP)
INTRODUCTION
Carbon fibers are obtained by pyrolysis of cellulose / Acrylonitrile in an inert atmosphere. It has much higher elasticity modulus than glass fibers & show better resistance to temperature & corrosive chemicals. However, they are short fibers & are more expensive. They use carbon fibers reinforced in polymer matrix like epoxy or polyester resins.
PROPERTIES
· Low density.
· Resistance to high temperature.
· Excellent resistance to corrosion.
APPLICATIONS
· They are used as structural components (like wings, body, stabilizers, etc.) ofaircrafts & helicopters.
· They are used in making sports goods (rackets, archery, racing bicycles, etc.),laptops, fishing rods, musical instruments, etc.
POLYHYDROXYBUTARATE-HYDROXYVALARATE (PHBV)
INTRODUCTION
It can be produced by Alcaligenes eutrophus when grown in the presence of glucose &either propanoic or valeric acid. PHBV is a copolymer of 3-hydroxybutanoic acid and 3-hydroxypentanoic acid. PHBV may also be synthesized from butyrolactone and valerolactone in the presence of oligomeric aluminoxane as catalyst. IUPAC Name: Poly(3-hydroxybutyrate-co-3-hydroxyvalerate)
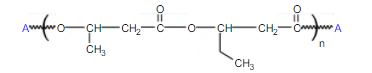
Repeating Unit of PHBV
PROPERTIES
· PHBV is a thermoplastic polymer. It is brittle, has low elongation at break and lowimpact resistance.
· Primitive mechanical properties.
· It is expensive and has low thermal stability
APPLICATIONS
· PHBV are used for controlled drug delivery as they are biocompatible &biodegradable. Also it is non-toxic.
· PHBV can be used as a sole structural material or as a part of degradablecomposites.
· PHBV can be used for films, blow molded bottles & as a coating on paper.
POLYCARBONATE (THERMOPLASTIC POLYMER)
INTRODUCTION
Polycarbonates (PC), known by the trademarked names Lexan, Makrolon, Makroclearand others, are a particular group of thermoplastic polymers. They are easily worked, molded, and thermoformed. Because of these properties, polycarbonates find many applications. It can be obtained by interaction of Bisphenol-A with diphenyl carbonate.
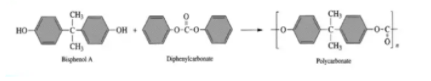
Preparation of Polycarbonate
PROPERTIES
· Its Tm is 265ºC.
· It has a good resistance (up to 140ºC) and has thermal stability.
· High impact strength & tensile strength.
· It has a tendency to yellow with long term ultraviolet exposure
APPLICATIONS
· They can be used for making electrical insulators, industrial plugs, sockets, switches,covers of cell phones, laptops, papers, etc.
· The production of CD, DVD’s, and Blue ray Disc by injection molding ofpolycarbonate.
· It is also used for widescreens for motorcycles, golf carts, small planes & helicopters.
· It can also be used for electronic display for use in mobile and portable device forsome LCD screens.
· It can be laminated to make bullet-proof glass
BIODEGRADEABLE POLYMER (POLYHYDROXYVALARATE)
INTRODUCTION
Biodegradable polymers are a specific type of polymer that breaks down after its intendedpurpose to result in natural byproducts such as gases (CO2, N2 ), water, biomass, and inorganic salts. These polymers are found both naturally and synthetically made, andlargely consist of ester, amide, and ether functional groups

Biodegradable Plastic
APPLICATIONS
· It can be used in food packing, foam for industrial packaging, film rapping, disposableplastic packing material such as single serve cups, disposable food service items,etc.
· Polymers like Polylactic acids are used in controlled drug delivery because ofbiocompatibility & biodegradability.
· Cell transplantation using biodegradable polymers scaffolds offers possibility tocreate completely natural new tissues & replace organ function.
· These polymers are used as time release coating for fertilizers & pesticides, makingfilms for moisture & heat retention
KEVLAR
INTRODUCTION
Kevlar (Poly paraphenylene terephthalamide) is a very high strength material that can be spun into ropes or fabric sheets that can be used as such or as an ingredient in composite material components.
The polymer has very high strength due to intermolecular hydrogen bond formed between carbonyl group and NH group

Kevlar
PROPERTIES
· It has outstanding high strength to weight ratio.
· It is thermally stable (M. P. > 500 oC).
· Crystalline density at 25oC is 1.23 g/cm3.
· Tg is 47oC and Tm is 220oC.
· It is very resistance to impact & abrasion damage
APPLICATIONS
· Kevlar is often combined with carbon fibers & embedded in epoxy resins to formhybrid composite that have the ability to withstand catastrophic impact.
· It can also be used in light weight boat hulls, high performance race cars.
· It can be used as cables for mooring lines, offshore drilling platforms, for parachutelines, fishing lines, mountaineering ropes & pulley ropes.
· It can be used in protective clothing & body armor
POLYHYDROXYBUTYRATE (PHB)
INTRODUCTION
Polyhydroxybutyrate (PHB) is a polyhydroxyalkanoate (PHA), a polymer belonging to thepolyesters class that are of interest as bio-derived and biodegradable plastics. The poly-3-hydroxybutyrate (P3HB) form of PHB is probably the most common type ofpolyhydroxyalkanoate, but other polymers of this class are produced by a variety oforganisms: these include poly-4-hydroxybutyrate (P4HB), polyhydroxyvalerate (PHV),polyhydroxyhexanoate (PHH), polyhydroxyoctanoate (PHO) and their copolymers.
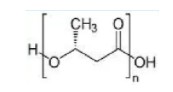
Polyhyroxybutyrate
PROPERTIES
· It has Tg 2ºC and Tm 175°C.
· Good ultra-violet resistance but poor resistance to acids and bases.
· Nontoxic.
APPLICATIONS
· PHB can be used for films, blow moulded bottles & as a coating on paper.
· PHB are used for controlled drug delivery as they are biocompatible &biodegradable. Also it is non-toxic.
· PHB can be used as a sole structural material or as a part of degradable composites
CONDUCTING POLYMER (POLYPYRROLE (PPY))
INTRODUCTION
Polypyrrole (PPy) is a type of organic polymer formed from by polymerization of pyrrole.Polypyrroles are conducting polymers, related members being polythiophene, polyaniline,and polyacetylene
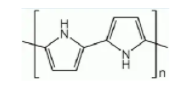
Molecular Structure of Polypyrrole
PROPERTIES
· They are amorphous, showing only weak diffraction.
· They are stable in air up to 150 °C
· PPy is an insulator
APPLICATIONS
· PPy and related conductive polymers have two main application in electronic devicesand for chemical sensors.
· PPy is also potential vehicle for drug delivery.
· The polymer matrix serves as a container for proteins