Basics of Polystyrene Production
Polystyrene is a widely used polymer. After production of the monomer, from one of a few processes, the monomer proceeds to further processing to form polystyrene.
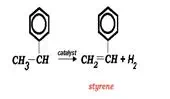
Styrene Monomer Production††††††††††††
The energy needed for the reaction is supplied by superheated steam (at about 720 ¬įC) that is injected into a vertically mounted fixed bed catalytic reactor with vaporized ethylbenzene.¬† The catalyst is iron oxide based and contains Cr2O3 and a potassium compound (KOH or K2CO3) which act as reaction promoters.¬
Typically, 2.5-3 kg steam are required for each kilogram of ethylbenzene to ensure sufficiently high temperatures throughout the reactor. ¬† The superheated steam supplies the necessary reaction temperature of 550-620 ¬įC throughout the reactor.¬† Ethylbenzene conversion is typically 60-65%.¬† Styrene selectivity is greater than 90%.¬† The three significant byproducts are toluene, benzene, and hydrogen.
After the reaction, the products are cooled rapidly (perhaps even quenched) to prevent polymerization.  The product stream (containing styrene, toulene, benzene, and unreacted ethylbenzene) is fractionally condensed after the hydrogen is flashed from the stream.  The hydrogen from the reaction is used as fuel to heat the steam (boiler fuel).  After adding a polymerization inhibitor (usually a phenol), the styrene is vacuum distilled in a series of four columns (often times packed columns) to reach the required 99.8% purity.  The separation is difficult due to the similar boiling points of styrene and ethylbenzene.   Typical capacity per plant ranges from 70,000 to 100,000 metric tonnes per year in each reactor and most plants contain multiple reactors or units.