
DEFINITION OF THE BASIC PVT PARAMETERS
The Pressure−Volume−Temperature relation for a real gas can be uniquely defined by the simple equation of state

in which the Z−factor, which accounts for the departure from ideal gas behaviour, can be determined as described in Chapter 1, sec. 5. Using this equation, it is a relatively simple matter to determine the relationship between surface volumes of gas and volumes in the reservoir as
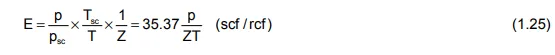
Unfortunately, no such simple equation of state exists which will describe the PVT properties of oil. Instead, several, so-called, PVT parameters must be measured by laboratory analysis of crude oil samples.
The parameters can then be used to express the relationship between surface and reservoir hydrocarbon volumes, equivalent to equ. (1.25). The complexity in relating surface volumes of hydrocarbon production to their equivalent volumes in the reservoir can be appreciated by considering fig.
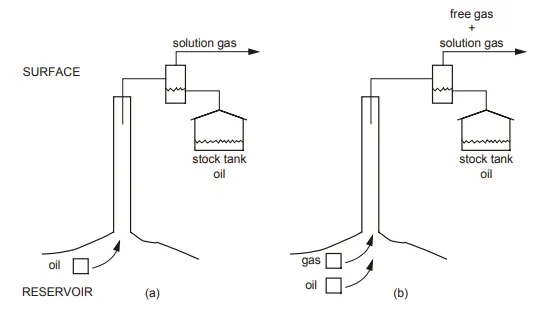
If the reservoir is below bubble point pressure, as depicted in fig. 2.1(b), the situation is more complicated. Now there are two hydrocarbon phases in the reservoir, gas saturated oil and liberated solution gas. During production to the surface, solution gas will be evolved from the oil phase and the total surface gas production will have two components; the gas which was free in the reservoir and the gas liberated from the oil during production. These separate components are indistinguishable at the surface and the problem is, therefore, how to divide the observed surface gas production into liberated and dissolved gas volumes in the reservoir.